SULIT 1. (a) A student observed the formation of white precipitate when he added copper(II) chloride and silver(1) nitrate in water. (i) Write a balance molecular equation for this reaction. (ii) Name the type of this reaction. (ii) Determine the spectator ion(s) in this reaction. (iv) If 2.00 g of copper(Il) chloride and 2.00 g of silver(1) nitrate were used in this reaction, determine the mass of the white solid formed. (v) If the student exchanges the silver(1) white precipitate. Explain your answer with suitable equation. rate with sodium nitrate as a reagent, determine if he can observe the formation of (b) Briefly describe how the atomic radii and ionization energies of group 1A(1) elements compare with those of group 8A(18). Explain why the values of these properties are so different between these two groups. Maximum file size: 4GB, maximum nunber of
SULIT 1. (a) A student observed the formation of white precipitate when he added copper(II) chloride and silver(1) nitrate in water. (i) Write a balance molecular equation for this reaction. (ii) Name the type of this reaction. (ii) Determine the spectator ion(s) in this reaction. (iv) If 2.00 g of copper(Il) chloride and 2.00 g of silver(1) nitrate were used in this reaction, determine the mass of the white solid formed. (v) If the student exchanges the silver(1) white precipitate. Explain your answer with suitable equation. rate with sodium nitrate as a reagent, determine if he can observe the formation of (b) Briefly describe how the atomic radii and ionization energies of group 1A(1) elements compare with those of group 8A(18). Explain why the values of these properties are so different between these two groups. Maximum file size: 4GB, maximum nunber of
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.58PAE
Related questions
Question
Transcribed Image Text:SULIT
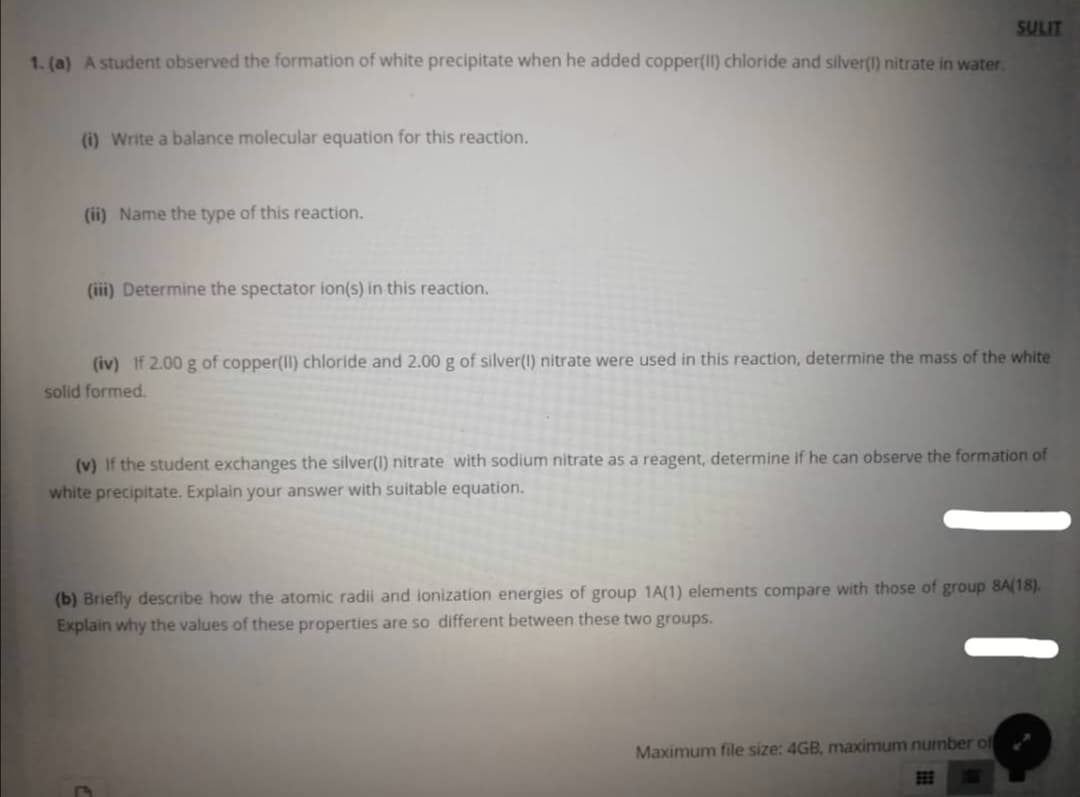
1. (a) A student observed the formation of white precipitate when he added copper(II) chloride and silver(1) nitrate in water.
(i) Write a balance molecular equation for this reaction.
(ii) Name the type of this reaction.
(ii) Determine the spectator ion(s) in this reaction.
(iv) If 2.00 g of copper(II) chloride and 2.00 g of silver(1) nitrate were used in this reaction, determine the mass of the white
solid formed.
(v) If the student exchanges the silver(1)
white precipitate. Explain your answer with suitable equation.
rate with sodium nitrate as a reagent, determine if he can observe the formation of
(b) Briefly describe how the atomic radii and ionization energies of group 1A(1) elements compare with those of group 8A(18).
Explain why the values of these properties are so different between these two groups.
Maximum file size: 4GB, maximum number of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
