Acetic acid and ethanol react to form ethyl acetate and water, like this: HCH,CO,(aq)+C,H,OH(aq)C,H,CO,CH3(aq)+H,O() Imagine 217. mmol of C,H,CO,CH, are removed from a flask containing a mixture of HCH,CO,, C,H,OH, C,H,CO, CH, and H,O at equilibrium, and then answer the following questions. O Zero. What is the rate of the reverse reaction before any CHsCO,CH3 has been removed from the flask? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction. O Zero. What is the rate of the reverse reaction just after the C,H5CO,CH3 has been removed from the flask? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction. O Zero. What is the rate of the reverse reaction when the system has again reached equilibrium? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction.
Acetic acid and ethanol react to form ethyl acetate and water, like this: HCH,CO,(aq)+C,H,OH(aq)C,H,CO,CH3(aq)+H,O() Imagine 217. mmol of C,H,CO,CH, are removed from a flask containing a mixture of HCH,CO,, C,H,OH, C,H,CO, CH, and H,O at equilibrium, and then answer the following questions. O Zero. What is the rate of the reverse reaction before any CHsCO,CH3 has been removed from the flask? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction. O Zero. What is the rate of the reverse reaction just after the C,H5CO,CH3 has been removed from the flask? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction. O Zero. What is the rate of the reverse reaction when the system has again reached equilibrium? O Greater than zero, but less than the rate of the forward reaction. O Greater than zero, and equal to the rate of the forward reaction. O Greater than zero, and greater than the rate of the forward reaction.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 120SCQ: In the reaction of two moles of gaseous hydrogen and one mole of gaseous oxygen to form two moles of...
Related questions
Question
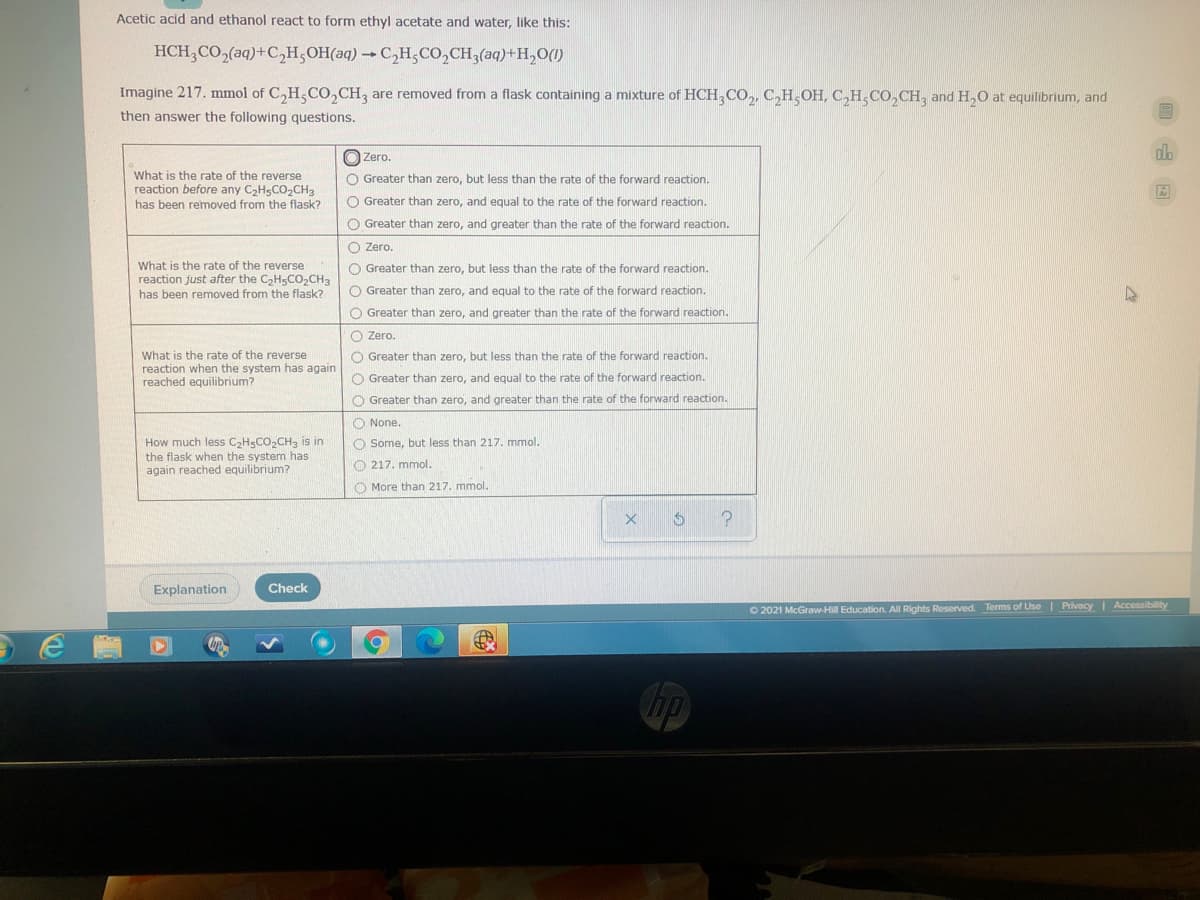
Transcribed Image Text:Acetic acid and ethanol react to form ethyl acetate and water, like this:
HCH,CO,(aq)+C2H;OH(aq) →C,H;CO,CH3(aq)+H,O()
Imagine 217. mmol of C,H,CO,CH, are removed from a flask containing a mixture of HCH,CO,, C,H,OH, C,H,CO,CH, and H,O at equilibrium, and
then answer the following questions.
O Zero.
What is the rate of the reverse
reaction before any C2H5CO,CH3
O Greater than zero, but less than the rate of the forward reaction.
has been removed from the flask?
O Greater than zero, and equal to the rate of the forward reaction.
O Greater than zero, and greater than the rate of the forward reaction.
O Zero.
What is the rate of the reverse
reaction just after the C,H5CO,CH3
has been removed from the flask?
O Greater than zero, but less than the rate of the forward reaction.
O Greater than zero, and equal to the rate of the forward reaction.
O Greater than zero, and greater than the rate of the forward reaction.
O Zero.
What is the rate of the reverse
reaction when the system has again
reached equilibrium?
O Greater than zero, but less than the rate of the forward reaction.
O Greater than zero, and equal to the rate of the forward reaction.
O Greater than zero, and greater than the rate of the forward reaction.
O None.
How much less C2HSCO2CH3 is in
the flask when the system has
again reached equilibrium?
O Some, but less than 217. mmol.
O 217. mmol.
O More than 217. mmol.
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use| Privacy I Accessibility
Chp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning