Addition of ammonium bisulfate In addition to the H,S already present in the flask, solid NH, HS is added until there is excess unreacted solid remaining. Part C What are the partial pressures of NH3 and H2S at equilibrium, that is, what are the values of PNH, and PH,S, respectively? Enter the partial pressure of ammonia followed by the partial pressure of hydrogen sulfide numerically in bars separated by a comma. • View Available Hint(s) ? PNH, , PH,s = bar Submit Part D What is the mole fraction, x, of H2S in the gas mixture at equilibrium? Express your answer numerically. • View Available Hint(s) να ΑΣφ ? XH,S = Submit
Addition of ammonium bisulfate In addition to the H,S already present in the flask, solid NH, HS is added until there is excess unreacted solid remaining. Part C What are the partial pressures of NH3 and H2S at equilibrium, that is, what are the values of PNH, and PH,S, respectively? Enter the partial pressure of ammonia followed by the partial pressure of hydrogen sulfide numerically in bars separated by a comma. • View Available Hint(s) ? PNH, , PH,s = bar Submit Part D What is the mole fraction, x, of H2S in the gas mixture at equilibrium? Express your answer numerically. • View Available Hint(s) να ΑΣφ ? XH,S = Submit
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 20QAP
Related questions
Question
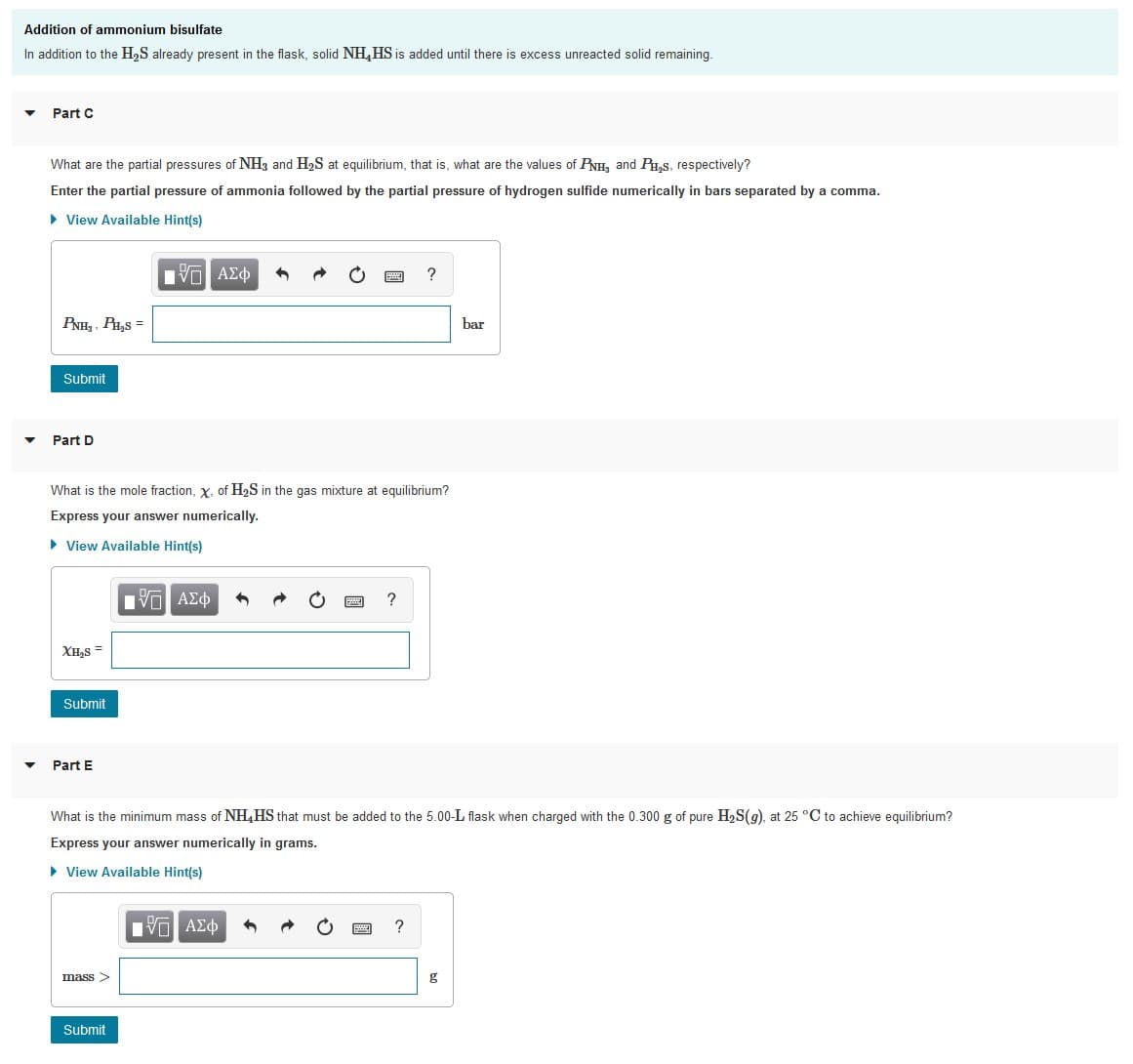
Transcribed Image Text:Addition of ammonium bisulfate
In addition to the H,S already present in the flask, solid NH,HS is added until there is excess unreacted solid remaining.
Part C
What are the partial pressures of NH3 and H2S at equilibrium, that is, what are the values of PNH, and P,s, respectively?
Enter the partial pressure of ammonia followed by the partial pressure of hydrogen sulfide numerically in bars separated by a comma.
• View Available Hint(s)
Πν ΑΣφ
?
PNH, , PH,S =
bar
Submit
Part D
What is the mole fraction, x, of H2S in the gas mixture at equilibrium?
Express your answer numerically.
• View Available Hint(s)
Πν ΑΣφ
XH,S =
Submit
Part E
What is the minimum mass of NH,HS that must be added to the 5.00-L flask when charged with the 0.300 g of pure H,S(9), at 25 °C to achieve equilibrium?
Express your answer numerically in grams.
• View Available Hint(s)
Vo AEO
?
mass>
g
Submit
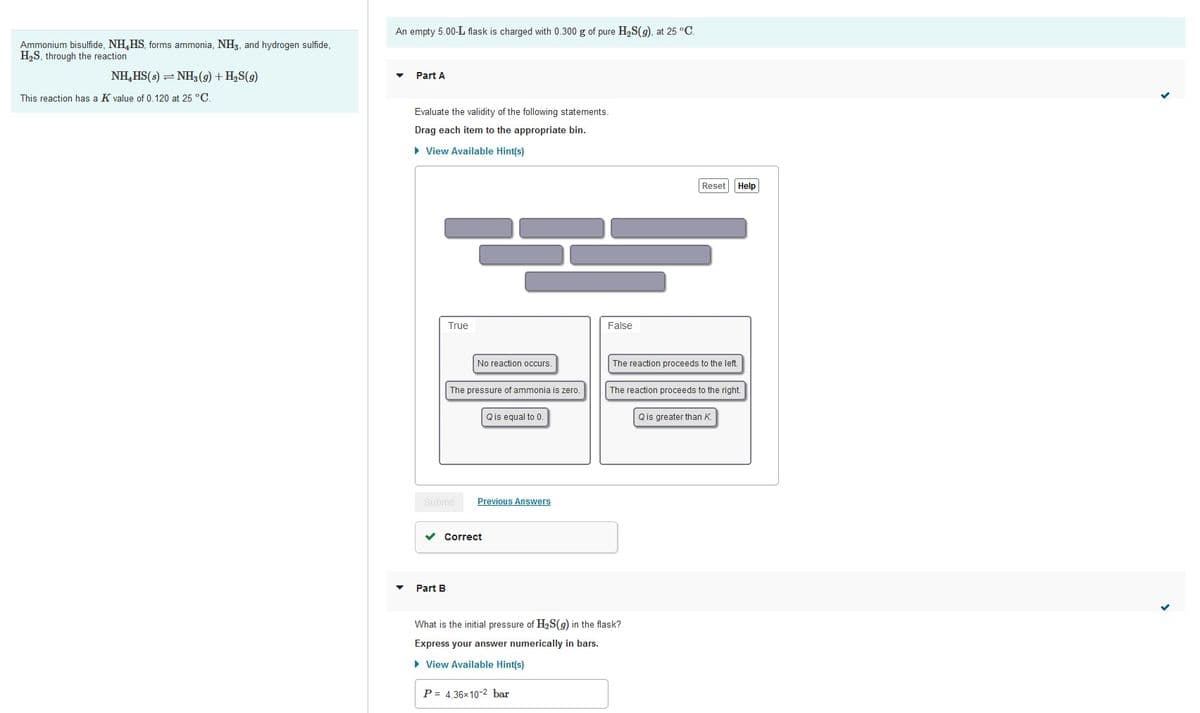
Transcribed Image Text:An empty 5.00-L flask is charged with 0.300 g of pure H,S(9), at 25 °C.
Ammonium bisulfide, NH,HS, forms ammonia, NH3, and hydrogen sulfide,
H2S, through the reaction
NH,HS(s) = NH3 (9) + H2S(g)
Part A
This reaction has a K value of 0.120 at 25 °C.
Evaluate the validity of the following statements.
Drag each item to the appropriate bin.
• View Available Hint(s)
Reset Help
Tru
False
No reaction occurs.
The reaction proceeds to the left.
The pressure of ammonia is zero.
The reaction proceeds to the right.
Qis equal to 0.
Qis greater than K.
Submit
Previous Answers
Correct
Part B
What is the initial pressure of H2S(9) in the flask?
Express your answer numerically in bars.
• View Available Hint(s)
P = 4.36x10-2 bar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
