AG", rzn =-474 kJ/mol Electrode Half-Reaction Standard Reduction Cathode O2(9) + 4 H* (aq) + 4 e → 2 H20(1) ? Anode 2 H* (aq) + 2 e- → H2(9) 0.00 e operation of a hydrogen fuel cell under standard conditions relies on the chemical reaction represented above. The table provides the relevan tentials. Based on the information given, which of the following equations can be used to calculate the standard reduction potential, in volts, o E red (cathode) = -474 4 x 96,500 A -474,000 в E red (cathode) = %3D 4 x 96,500 4 x 96,500 E red (cathode) 474 4 x 96,500 E red (cathode) = -474,000 D.
AG", rzn =-474 kJ/mol Electrode Half-Reaction Standard Reduction Cathode O2(9) + 4 H* (aq) + 4 e → 2 H20(1) ? Anode 2 H* (aq) + 2 e- → H2(9) 0.00 e operation of a hydrogen fuel cell under standard conditions relies on the chemical reaction represented above. The table provides the relevan tentials. Based on the information given, which of the following equations can be used to calculate the standard reduction potential, in volts, o E red (cathode) = -474 4 x 96,500 A -474,000 в E red (cathode) = %3D 4 x 96,500 4 x 96,500 E red (cathode) 474 4 x 96,500 E red (cathode) = -474,000 D.
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 115AE: The saturated calomel electrode. abbreviated SCE. is often used as a reference electrode in making...
Related questions
Question
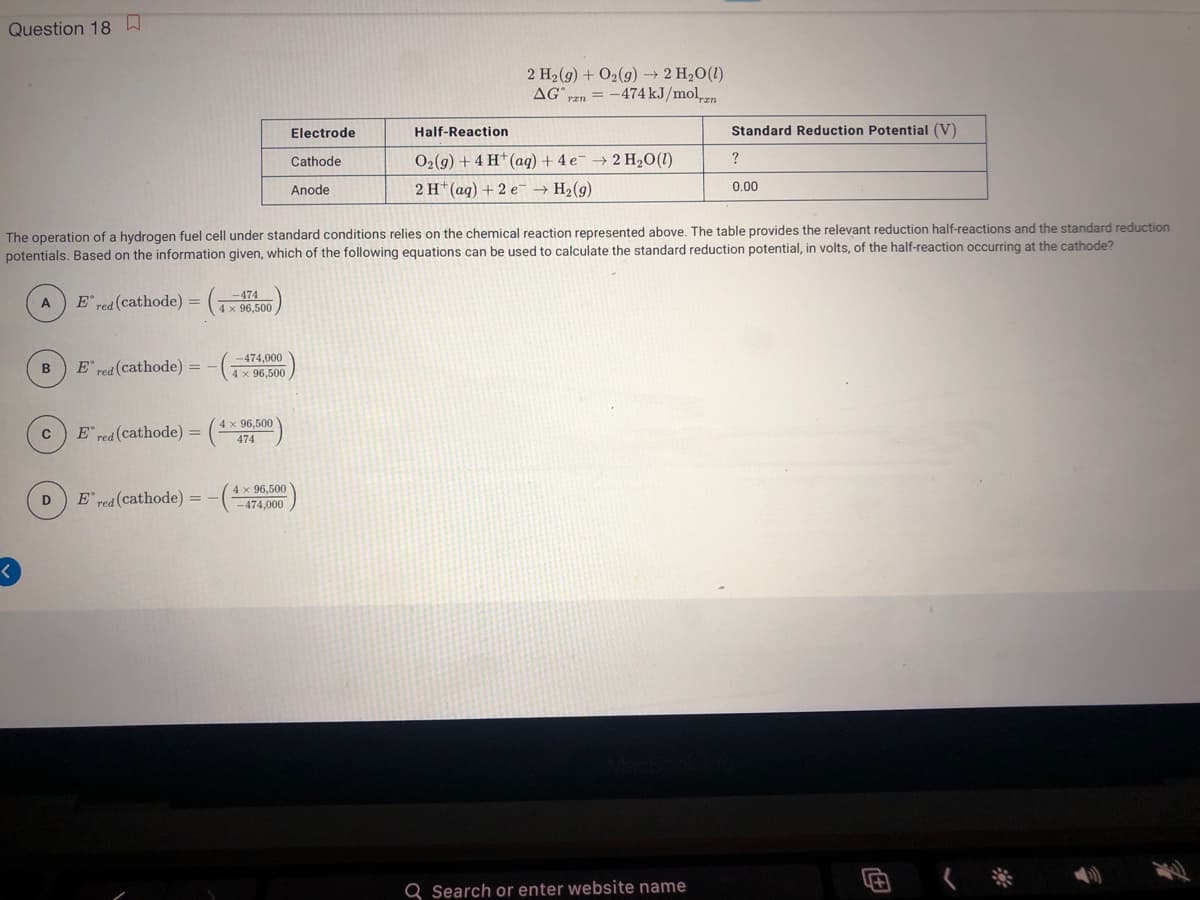
Transcribed Image Text:Question 18A
2 H2(g) + 02(g) → 2 H2O(1)
AGan = -474 kJ/mol
Electrode
Half-Reaction
Standard Reduction Potential (V)
Cathode
O2(9) + 4 H*(aq) + 4 e- → 2 H2O(1)
?
Anode
2 H*(ag) + 2 e → H2(g)
0.00
The operation of a hydrogen fuel cell under standard conditions relies on the chemical reaction represented above. The table provides the relevant reduction half-reactions and the standard reduction
potentials. Based on the information given, which of the following equations can be used to calculate the standard reduction potential, in volts, of the half-reaction occurring at the cathode?
E red (cathode)
-474
4 x 96,500
A
E' red (cathode)
-474,000
4 x 96,500
E red (cathode) =
4 x 96,500
474
4 x 96,500
D
E' red (cathode) =
-474,000
回く*
Q Search or enter website name
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning