Again, focusing on the first nuclear equation shown below (vanadium on the left side of the arrow, chromium and beta particle on the right). Which expression below best explains why the top numbers (total baryons) on both sides of the arrow are balanced? Name Symbol(s) Representation Description Nuclear Equation fill in the gaps He or fa (High-energy) helium nuclei consisting of two protons and two neutrons "y→,Cr+ B 7Ge→, Ga+e* Alpha particle Beta particle fe or B (High-energy) electrons V→"Ti+ "C→"N+ fe or B Particles with the same mass as an electron but with 1 unit of positive charge Positron 105 105 Sn→+ 92 90 H or tp 50 50 Proton Nuclei of hydrogen atoms Th Th+y 210 Po- Pb+ a Particles with a mass approximately equal to that of a proton but with no charge Neutron 51 = 52 + (-1) 51 = 51 + 0 51 50 + 1
Again, focusing on the first nuclear equation shown below (vanadium on the left side of the arrow, chromium and beta particle on the right). Which expression below best explains why the top numbers (total baryons) on both sides of the arrow are balanced? Name Symbol(s) Representation Description Nuclear Equation fill in the gaps He or fa (High-energy) helium nuclei consisting of two protons and two neutrons "y→,Cr+ B 7Ge→, Ga+e* Alpha particle Beta particle fe or B (High-energy) electrons V→"Ti+ "C→"N+ fe or B Particles with the same mass as an electron but with 1 unit of positive charge Positron 105 105 Sn→+ 92 90 H or tp 50 50 Proton Nuclei of hydrogen atoms Th Th+y 210 Po- Pb+ a Particles with a mass approximately equal to that of a proton but with no charge Neutron 51 = 52 + (-1) 51 = 51 + 0 51 50 + 1
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter19: Radioactivity And Nuclear Energy
Section: Chapter Questions
Problem 11A
Related questions
Question
100%
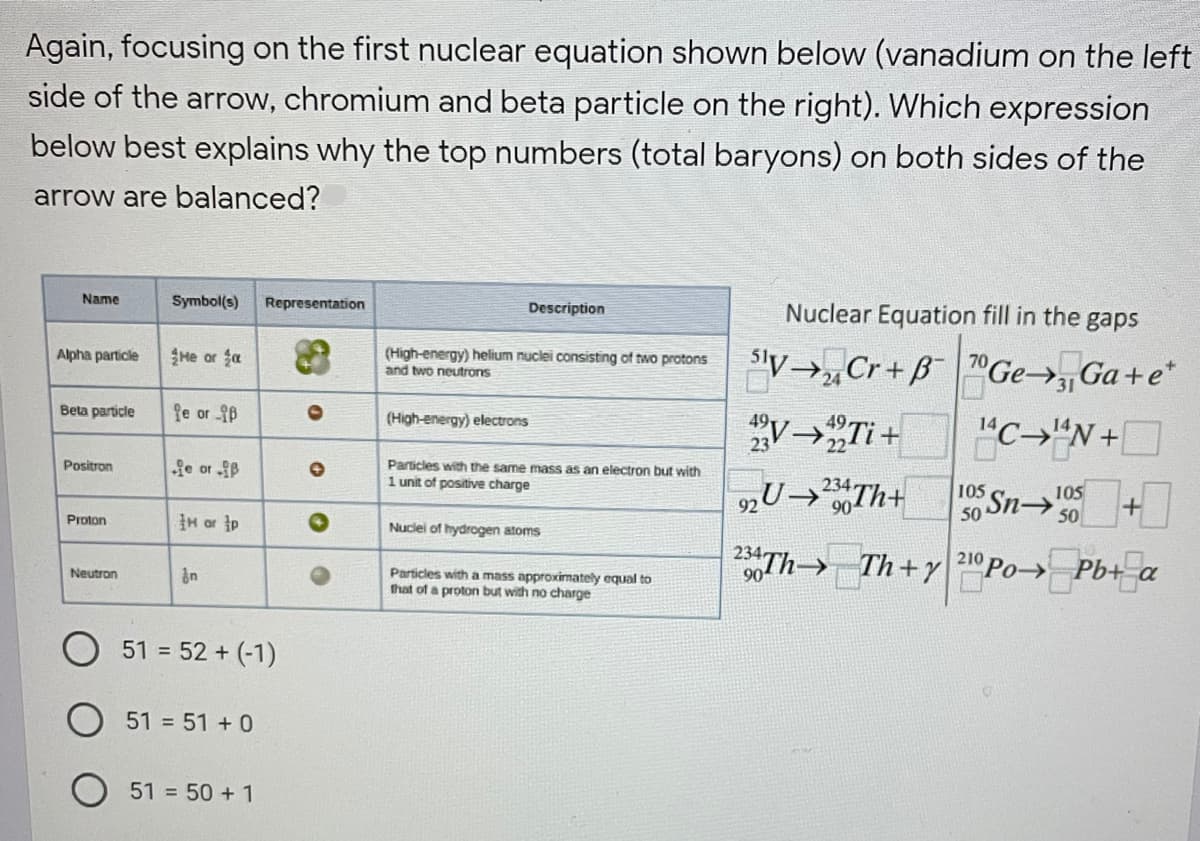
Transcribed Image Text:Again, focusing on the first nuclear equation shown below (vanadium on the left
side of the arrow, chromium and beta particle on the right). Which expression
below best explains why the top numbers (total baryons) on both sides of the
arrow are balanced?
Name
Symbol(s)
Representation
Description
Nuclear Equation fill in the gaps
He or fa
(High-energy) helium nuclei consisting of two protons
and two neutrons
'y→,Cr+ B Ge→ Ga+e*
Alpha particle
24
Beta particle
fe or B
(High-energy) electrons
V→„Ti+
"C→"N+
fe or B
Particles with the same mass as an electron but with
1 unit of positive charge
Positron
Sn +
Th Th+y 210 Po Pb+ a
105
105
90
H or p
50
50
Proton
Nuclei of hydrogen atoms
->
Particles with a mass approximately equal to
that of a proton but with no charge
Neutron
51 = 52 + (-1)
51 = 51 + 0
51 = 50 + 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning