Air is about 78.0% nitrogen molecules and 21.0% oxygen molecules. Several other gases make up the remaining 19% of air molecules. Part A What is the partial pressure of nitrogen in air at atmospheric pressure (101 kPa)? Assume ideal behaviour. Express your answer to three significant figures and include the appropriate units.
Air is about 78.0% nitrogen molecules and 21.0% oxygen molecules. Several other gases make up the remaining 19% of air molecules. Part A What is the partial pressure of nitrogen in air at atmospheric pressure (101 kPa)? Assume ideal behaviour. Express your answer to three significant figures and include the appropriate units.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
SectionU3.10: Feeling Under Pressure: Boyle's Law
Problem 7E
Related questions
Question
Transcribed Image Text:Review | Constants | Periodic Table
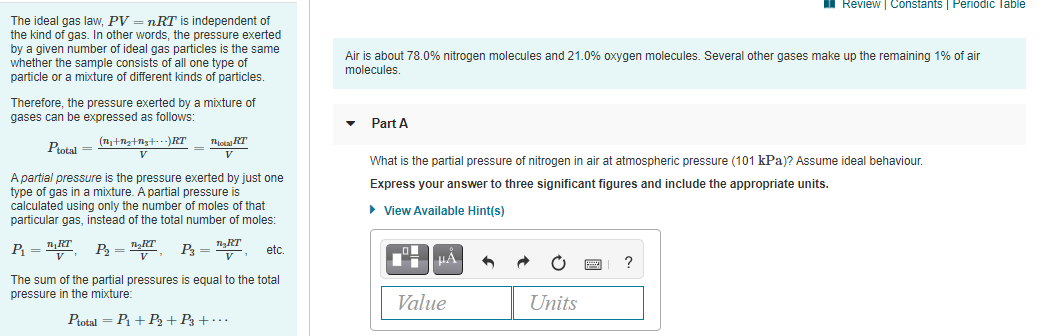
The ideal gas law, PV = nRT is independent of
the kind of gas. In other words, the pressure exerted
by a given number of ideal gas particles is the same
whether the sample consists of all one type of
particle or a mixture of different kinds of particles.
Air is about 78.0% nitrogen molecules and 21.0% oxygen molecules. Several other gases make up the remaining 1% of air
molecules.
Therefore, the pressure exerted by a mixture of
gases can be expressed as follows:
Part A
Ptotal =
Tota RT
V
What is the partial pressure of nitrogen in air at atmospheric pressure (101 kPa)? Assume ideal behaviour.
A partial pressure is the pressure exerted by just one
type of gas in a mixture. A partial pressure is
calculated using only the number of moles of that
particular gas, instead of the total number of moles:
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
P = "
TgRT
P3 =v
etc.
HA
The sum of the partial pressures is equal to the total
pressure in the mixture:
Value
Units
Ptotal = P1 + P2 + P3 + ·
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning