a)Is it possible to have an ionic compound that is not water soluble?b) Is it possible for an electrolyte to be covalent?
Like dissolves like.
That means polar and ionic compounds are soluble in polar solvents
and non-polar substances are soluble in non-polar solvents.
a)
Water is a polar solvent.
The structure of water is shown below:
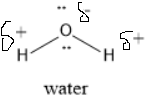
Oxygen attains partial negative charge due to its high electronegativity.
Hydrogen attains partial positive charge.
Due to this water attains polar nature.
All ionic compounds ionize completely in water.
These positive ions are attracted to partially negative oxygen and the negative ions are attracted to partially positive hydrogen of water molecule.
So, there are no ionic compounds that are not soluble in water.
All ionic compounds are soluble in water.
Step by step
Solved in 4 steps with 1 images









