Aldol Condensation Introduction As you've learned in lecture, a condensation reaction is one where two molecules are joined together while expelling another small molecule such as water or an alcohol in the process. Many condensations involve the alpha carbon of one carbonyl compound adding to another carbonyl group. This is classification of reactions is known as the aldol condensation. The reaction we'll investigate is generally known as a mixed aldol condensation and more specifically a Claisen-Schmidt reaction. This occurs when an aldehyde is reacted with a ketone upon the addition of sodium hydroxide functioning as a base. The product, dibenzalacetone, is prepared by condensation of acetone with two equivalents of benzaldehyde. The aldehyde carbonyl is more reactive than the ketone carbonyl in the acetone. This means that the aldehyde will react more rapidly with the anion of the ketone to produce the ẞ-hydroxy ketone, which will then undergo base-catalyzed dehydration. Depending on the ratio of reactants, the reaction will either give mono or dibenzalacetone. In this reaction, enough solvent, ethanol, is present to dissolve both the starting material (benzaldehyde) and the intermediate (benzalacetone). Once the intermediate has formed due to the first addition, another equivalent of benzaldehyde can be added to yield the product dibenzalacetone. This product is conveniently insoluble in aqueous ethanol and precipitates out of the reaction mixture once it has formed. Both the starting material and the intermediate are soluble in aqueous ethanol. 2 H NaOH H₂O ethanol acetone benzaldehyde Figure 1: General reaction scheme for the aldol condensation. dibenzalacetone The aldol condensation begins when the acidic proton is removed from the alpha carbon of the carbonyl. This generates a powerful nucleophile, an enolate. The enolate will then attack the carbonyl carbon. The reaction involves several general steps: 1. base-catalyzed generation of the enolate 2. nucleophilic attack of the enolate on a carbonyl carbon 3. protonation of the resulting anion to give the initial aldol product, an α, ß -hydroxy carbonyl compound. This reaction exists in equilibrium and is reversible. The a, ẞ -hydroxy carbonyl can be isolated because the next dehydration step is generally slower than the initial addition reaction. The final step of the reaction is a hydroxide catalyzed dehydration of the product by way of its enolate. We know that typically a hydroxide ion is not a great leaving group, however in this case the alpha hydrogen to the ketone is acidic and can be removed under strongly basic conditions. The elimination step also produces a stable conjugated α, ß -unsaturated ketone. Because of the conditions and these two factors, the hydroxide is able to function as a leaving group. :O: my of oth H H H надн TOH O H-OH :OH Figure 2: Mechanism for the formation of dibenzalacetone. H There are three isomeric dibenzalacetones, each with a different melting point. One melts at 110°C, another at 60 °C and the final one is a liquid. In the final step of the aldol condensation, loss of water from the ẞ -hydroxy ketone can form molecules in which the alkene hydrogen atoms are either cis or trans to another. Thus, along with the melting point, the 1H NMR spectrum provides the necessary evidence for the stereochemistry of the product. The J value or coupling constant of cis protons for alkenes is 10-12 Hz, whereas the J value of trans protons is 15 20 Hz. Experimental Using a syringe, measure 1.2 mL of benzaldehyde. Add this to a 50 mL beaker along with the theoretical quantity of acetone needed (see prelab question #2) and set aside. Next, add 1.2 g of sodium hydroxide to 12 mL of water and 10 mL of ethanol in a 100 mL beaker. Add a stir bar and allow it to stir on a stir plate until the sodium hydroxide is completely dissolved. Once completely dissolved, add half of the acetone/benzaldehyde mixture to the sodium hydroxide
Aldol Condensation Introduction As you've learned in lecture, a condensation reaction is one where two molecules are joined together while expelling another small molecule such as water or an alcohol in the process. Many condensations involve the alpha carbon of one carbonyl compound adding to another carbonyl group. This is classification of reactions is known as the aldol condensation. The reaction we'll investigate is generally known as a mixed aldol condensation and more specifically a Claisen-Schmidt reaction. This occurs when an aldehyde is reacted with a ketone upon the addition of sodium hydroxide functioning as a base. The product, dibenzalacetone, is prepared by condensation of acetone with two equivalents of benzaldehyde. The aldehyde carbonyl is more reactive than the ketone carbonyl in the acetone. This means that the aldehyde will react more rapidly with the anion of the ketone to produce the ẞ-hydroxy ketone, which will then undergo base-catalyzed dehydration. Depending on the ratio of reactants, the reaction will either give mono or dibenzalacetone. In this reaction, enough solvent, ethanol, is present to dissolve both the starting material (benzaldehyde) and the intermediate (benzalacetone). Once the intermediate has formed due to the first addition, another equivalent of benzaldehyde can be added to yield the product dibenzalacetone. This product is conveniently insoluble in aqueous ethanol and precipitates out of the reaction mixture once it has formed. Both the starting material and the intermediate are soluble in aqueous ethanol. 2 H NaOH H₂O ethanol acetone benzaldehyde Figure 1: General reaction scheme for the aldol condensation. dibenzalacetone The aldol condensation begins when the acidic proton is removed from the alpha carbon of the carbonyl. This generates a powerful nucleophile, an enolate. The enolate will then attack the carbonyl carbon. The reaction involves several general steps: 1. base-catalyzed generation of the enolate 2. nucleophilic attack of the enolate on a carbonyl carbon 3. protonation of the resulting anion to give the initial aldol product, an α, ß -hydroxy carbonyl compound. This reaction exists in equilibrium and is reversible. The a, ẞ -hydroxy carbonyl can be isolated because the next dehydration step is generally slower than the initial addition reaction. The final step of the reaction is a hydroxide catalyzed dehydration of the product by way of its enolate. We know that typically a hydroxide ion is not a great leaving group, however in this case the alpha hydrogen to the ketone is acidic and can be removed under strongly basic conditions. The elimination step also produces a stable conjugated α, ß -unsaturated ketone. Because of the conditions and these two factors, the hydroxide is able to function as a leaving group. :O: my of oth H H H надн TOH O H-OH :OH Figure 2: Mechanism for the formation of dibenzalacetone. H There are three isomeric dibenzalacetones, each with a different melting point. One melts at 110°C, another at 60 °C and the final one is a liquid. In the final step of the aldol condensation, loss of water from the ẞ -hydroxy ketone can form molecules in which the alkene hydrogen atoms are either cis or trans to another. Thus, along with the melting point, the 1H NMR spectrum provides the necessary evidence for the stereochemistry of the product. The J value or coupling constant of cis protons for alkenes is 10-12 Hz, whereas the J value of trans protons is 15 20 Hz. Experimental Using a syringe, measure 1.2 mL of benzaldehyde. Add this to a 50 mL beaker along with the theoretical quantity of acetone needed (see prelab question #2) and set aside. Next, add 1.2 g of sodium hydroxide to 12 mL of water and 10 mL of ethanol in a 100 mL beaker. Add a stir bar and allow it to stir on a stir plate until the sodium hydroxide is completely dissolved. Once completely dissolved, add half of the acetone/benzaldehyde mixture to the sodium hydroxide
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 63EQ
Related questions
Question
Which side product could be expected in this reaction if the product of the first addition, benzalacetone, reacts with acetone instead of the benzaldehyde? please show the structure of the side product and the mechanistic step(s) showing how it is formed. thanks in advance
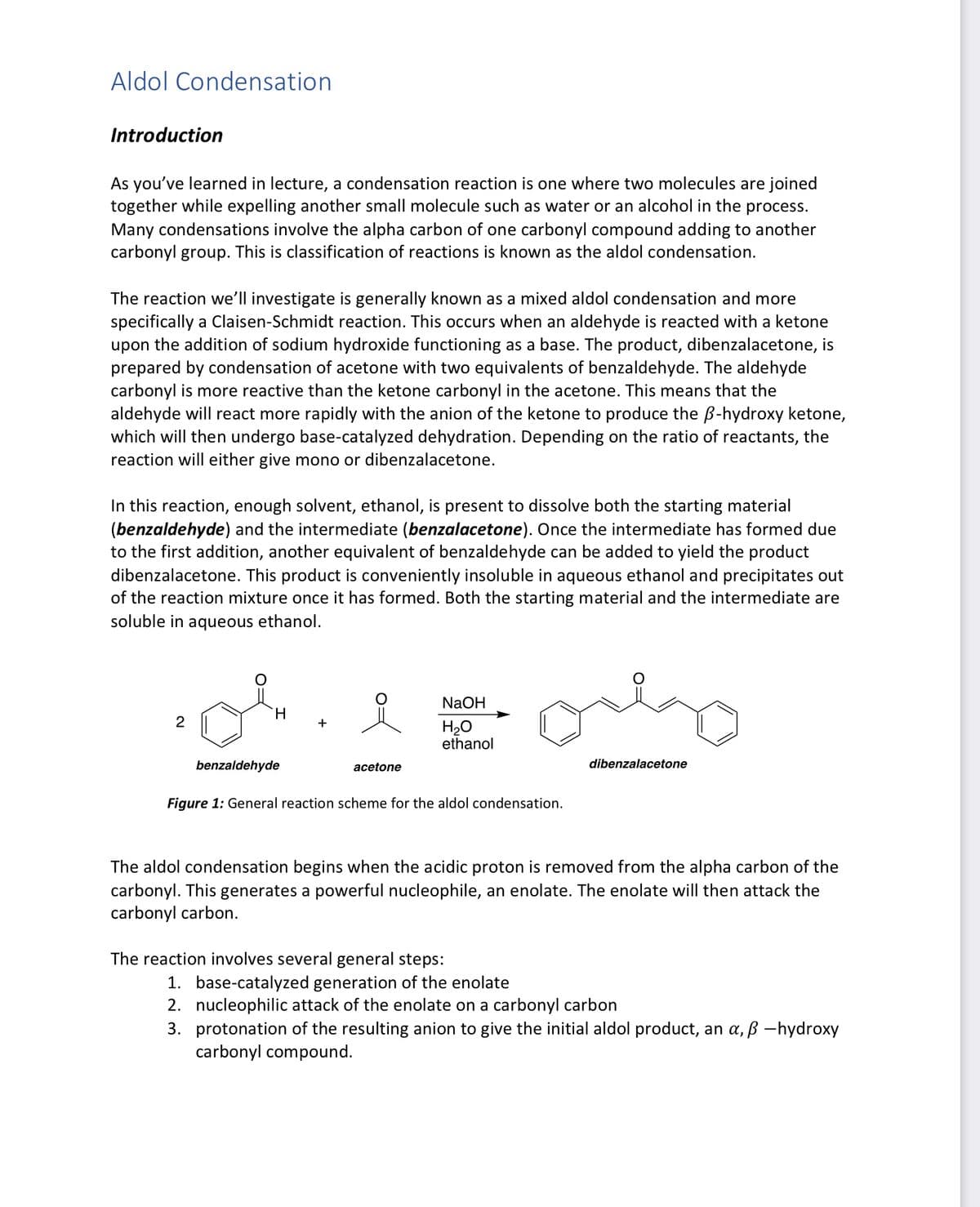
Transcribed Image Text:Aldol Condensation
Introduction
As you've learned in lecture, a condensation reaction is one where two molecules are joined
together while expelling another small molecule such as water or an alcohol in the process.
Many condensations involve the alpha carbon of one carbonyl compound adding to another
carbonyl group. This is classification of reactions is known as the aldol condensation.
The reaction we'll investigate is generally known as a mixed aldol condensation and more
specifically a Claisen-Schmidt reaction. This occurs when an aldehyde is reacted with a ketone
upon the addition of sodium hydroxide functioning as a base. The product, dibenzalacetone, is
prepared by condensation of acetone with two equivalents of benzaldehyde. The aldehyde
carbonyl is more reactive than the ketone carbonyl in the acetone. This means that the
aldehyde will react more rapidly with the anion of the ketone to produce the ẞ-hydroxy ketone,
which will then undergo base-catalyzed dehydration. Depending on the ratio of reactants, the
reaction will either give mono or dibenzalacetone.
In this reaction, enough solvent, ethanol, is present to dissolve both the starting material
(benzaldehyde) and the intermediate (benzalacetone). Once the intermediate has formed due
to the first addition, another equivalent of benzaldehyde can be added to yield the product
dibenzalacetone. This product is conveniently insoluble in aqueous ethanol and precipitates out
of the reaction mixture once it has formed. Both the starting material and the intermediate are
soluble in aqueous ethanol.
2
H
NaOH
H₂O
ethanol
acetone
benzaldehyde
Figure 1: General reaction scheme for the aldol condensation.
dibenzalacetone
The aldol condensation begins when the acidic proton is removed from the alpha carbon of the
carbonyl. This generates a powerful nucleophile, an enolate. The enolate will then attack the
carbonyl carbon.
The reaction involves several general steps:
1. base-catalyzed generation of the enolate
2. nucleophilic attack of the enolate on a carbonyl carbon
3. protonation of the resulting anion to give the initial aldol product, an α, ß -hydroxy
carbonyl compound.
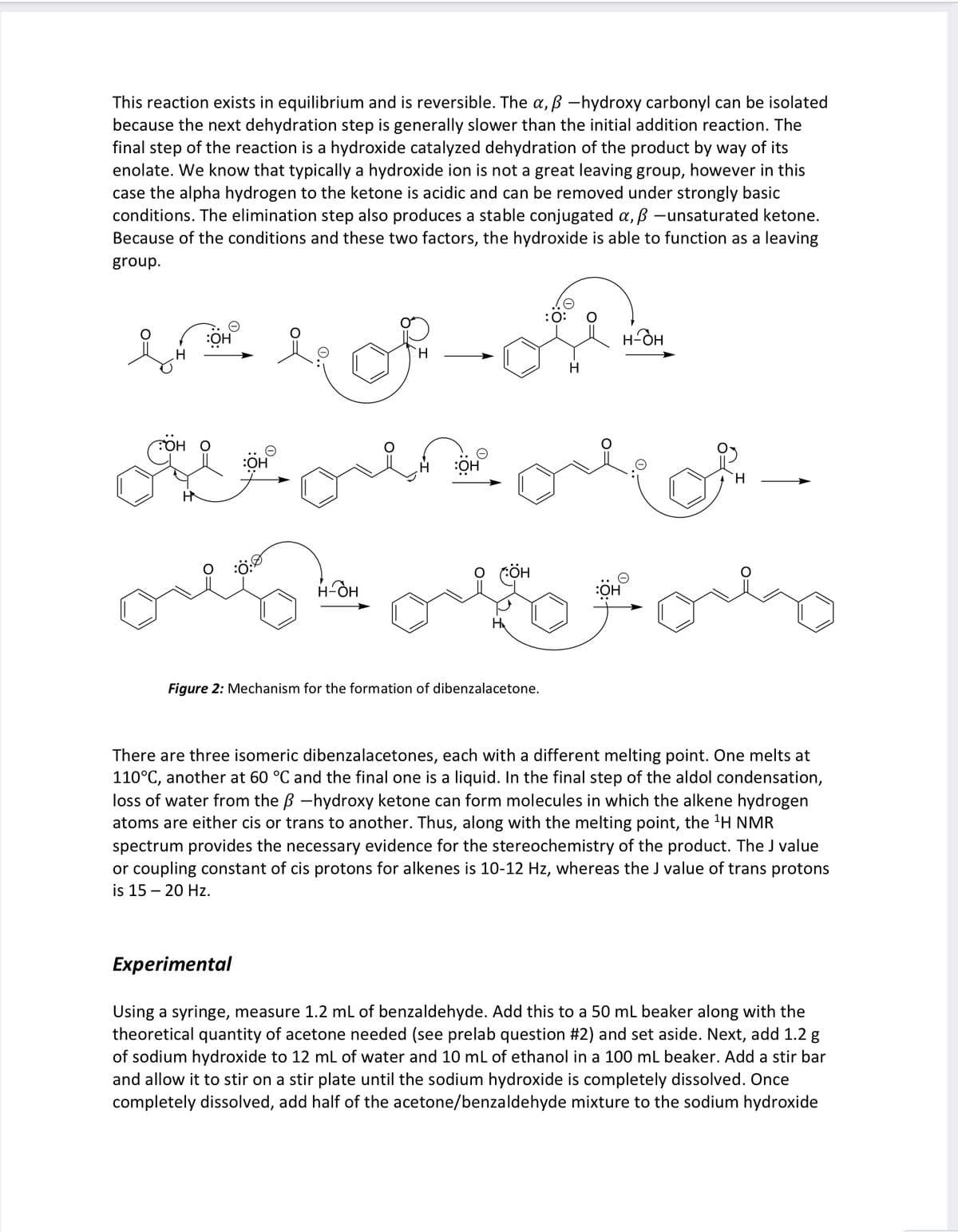
Transcribed Image Text:This reaction exists in equilibrium and is reversible. The a, ẞ -hydroxy carbonyl can be isolated
because the next dehydration step is generally slower than the initial addition reaction. The
final step of the reaction is a hydroxide catalyzed dehydration of the product by way of its
enolate. We know that typically a hydroxide ion is not a great leaving group, however in this
case the alpha hydrogen to the ketone is acidic and can be removed under strongly basic
conditions. The elimination step also produces a stable conjugated α, ß -unsaturated ketone.
Because of the conditions and these two factors, the hydroxide is able to function as a leaving
group.
:O:
my of oth
H
H
H
надн
TOH O
H-OH
:OH
Figure 2: Mechanism for the formation of dibenzalacetone.
H
There are three isomeric dibenzalacetones, each with a different melting point. One melts at
110°C, another at 60 °C and the final one is a liquid. In the final step of the aldol condensation,
loss of water from the ẞ -hydroxy ketone can form molecules in which the alkene hydrogen
atoms are either cis or trans to another. Thus, along with the melting point, the 1H NMR
spectrum provides the necessary evidence for the stereochemistry of the product. The J value
or coupling constant of cis protons for alkenes is 10-12 Hz, whereas the J value of trans protons
is 15 20 Hz.
Experimental
Using a syringe, measure 1.2 mL of benzaldehyde. Add this to a 50 mL beaker along with the
theoretical quantity of acetone needed (see prelab question #2) and set aside. Next, add 1.2 g
of sodium hydroxide to 12 mL of water and 10 mL of ethanol in a 100 mL beaker. Add a stir bar
and allow it to stir on a stir plate until the sodium hydroxide is completely dissolved. Once
completely dissolved, add half of the acetone/benzaldehyde mixture to the sodium hydroxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 2 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning