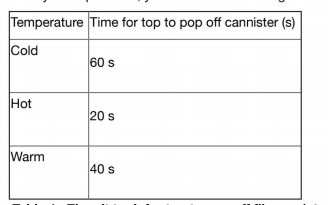
Alka-seltzer is a tablet that people take to control acid indigestion. When it is added to water, Alka-seltzer produces carbon dioxide gas. This gas can produce pressure, causing the top to pop off a film cannister in a specific period of time. Your investigation involves determining how the temperature of water will affect the rate at which carbon dioxide gas forms. The materials that are available to you are: film canister with top, thermometer, water, kettle, ice, Alka-Seltzer tablets, timer a. Hypothesize how temperature will affect the rate at which carbon dioxide forms. (1:1) Give specific reasons for your answer (A:3) b. Based upon the materials listed above, design an experiment to fairly test your hypothesis. Ensure you number your steps. (1:2, C:2) From your experiment, you collect the following data: Temperature Time for top to pop off cannister (s) Cold 60 s Hot 20 s Warm 40 s Table 1: Time it took for top to pop off film canister after Alka-seltzer has been added to water. c. Do the observations from this experiment agree or disagree with your hypothesis? Provide experimental evidence to validate your answer. (1:3, C:2) d. What do you think would happen if the concentration of alka-seltzer in each trial was decreased? Be specific in your answer. (A:3)
Alka-seltzer is a tablet that people take to control acid indigestion. When it is added to water, Alka-seltzer produces carbon dioxide gas. This gas can produce pressure, causing the top to pop off a film cannister in a specific period of time. Your investigation involves determining how the temperature of water will affect the rate at which carbon dioxide gas forms. The materials that are available to you are: film canister with top, thermometer, water, kettle, ice, Alka-Seltzer tablets, timer a. Hypothesize how temperature will affect the rate at which carbon dioxide forms. (1:1) Give specific reasons for your answer (A:3) b. Based upon the materials listed above, design an experiment to fairly test your hypothesis. Ensure you number your steps. (1:2, C:2) From your experiment, you collect the following data: Temperature Time for top to pop off cannister (s) Cold 60 s Hot 20 s Warm 40 s Table 1: Time it took for top to pop off film canister after Alka-seltzer has been added to water. c. Do the observations from this experiment agree or disagree with your hypothesis? Provide experimental evidence to validate your answer. (1:3, C:2) d. What do you think would happen if the concentration of alka-seltzer in each trial was decreased? Be specific in your answer. (A:3)
Trending now
This is a popular solution!
Step by step
Solved in 4 steps









