CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS O Previous Page 18 of 36 Nex o A closed flask contains a 0.25 moles of O2 which exerts a pressure of 0.50 atm. If 0.75 moles of CO, is added to the container what is the total pressure in the flask? Previous Page 17 of 36 Nexo References Use the References to access important values if needed for this question. mixture of H2 and Ne is placed in a 2.00-L flask at 25 °C. The partial pressure of the H, is 1.3 atm, and the partial pressure of the Ne is 2.7 atm. What is the mole fraction of H2?
CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS O Previous Page 18 of 36 Nex o A closed flask contains a 0.25 moles of O2 which exerts a pressure of 0.50 atm. If 0.75 moles of CO, is added to the container what is the total pressure in the flask? Previous Page 17 of 36 Nexo References Use the References to access important values if needed for this question. mixture of H2 and Ne is placed in a 2.00-L flask at 25 °C. The partial pressure of the H, is 1.3 atm, and the partial pressure of the Ne is 2.7 atm. What is the mole fraction of H2?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter9: The Gaseous State
Section: Chapter Questions
Problem 22P
Related questions
Question
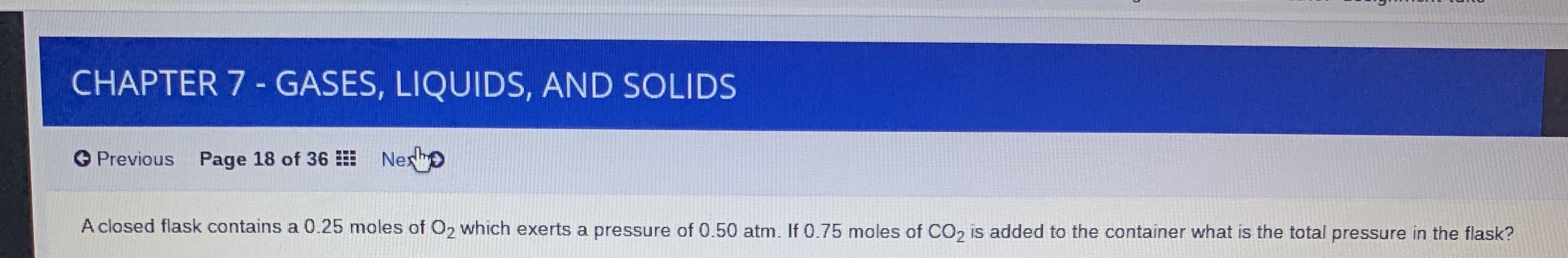
Transcribed Image Text:CHAPTER 7 - GASES, LIQUIDS, AND SOLIDS
O Previous Page 18 of 36 Nex o
A closed flask contains a 0.25 moles of O2 which exerts a pressure of 0.50 atm. If 0.75 moles of CO, is added to the container what is the total pressure in the flask?
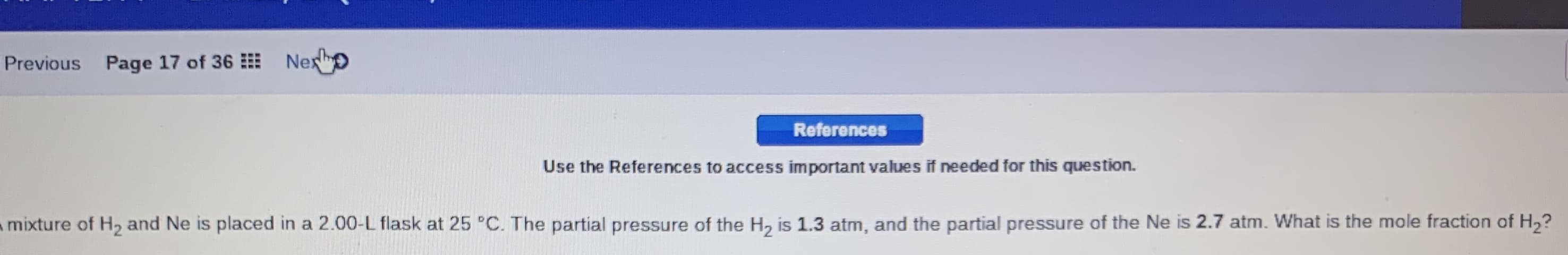
Transcribed Image Text:Previous Page 17 of 36 Nexo
References
Use the References to access important values if needed for this question.
mixture of H2 and Ne is placed in a 2.00-L flask at 25 °C. The partial pressure of the H, is 1.3 atm, and the partial pressure of the Ne is 2.7 atm. What is the mole fraction of H2?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning