Alkyl diazonium salts are unstable even at low temperature. They decompose to form carbocations, which go on to form products of substitution, elimination, and (sometimes) rearrangement. Keeping this in mind, draw a stepwise mechanism that forms all of the following products. NANO2 он + HCI, H2O NH2 он
Alkyl diazonium salts are unstable even at low temperature. They decompose to form carbocations, which go on to form products of substitution, elimination, and (sometimes) rearrangement. Keeping this in mind, draw a stepwise mechanism that forms all of the following products. NANO2 он + HCI, H2O NH2 он
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter26: Aldol And Claisen Reactions
Section: Chapter Questions
Problem 26E
Related questions
Question
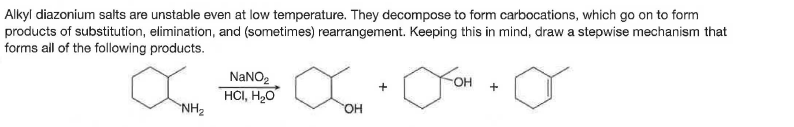
Transcribed Image Text:Alkyl diazonium salts are unstable even at low temperature. They decompose to form carbocations, which go on to form
products of substitution, elimination, and (sometimes) rearrangement. Keeping this in mind, draw a stepwise mechanism that
forms all of the following products.
NANO2
он
+
HCI, H2O
NH2
он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
