All of the following alcohols have the same molecular formula (C5H120), but they have significantly different boiling points. Explain why. OH НО ОН 2-Methylbutan-2-ol Boiling point = 102 °C Pentan-1-ol Pentan-3-ol Boiling point = 136–138 °C Boiling point = 114–115 °C
All of the following alcohols have the same molecular formula (C5H120), but they have significantly different boiling points. Explain why. OH НО ОН 2-Methylbutan-2-ol Boiling point = 102 °C Pentan-1-ol Pentan-3-ol Boiling point = 136–138 °C Boiling point = 114–115 °C
Organic And Biological Chemistry
7th Edition
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:STOKER, H. Stephen (howard Stephen)
Chapter6: Amines And Amides
Section: Chapter Questions
Problem 6.38EP
Related questions
Question
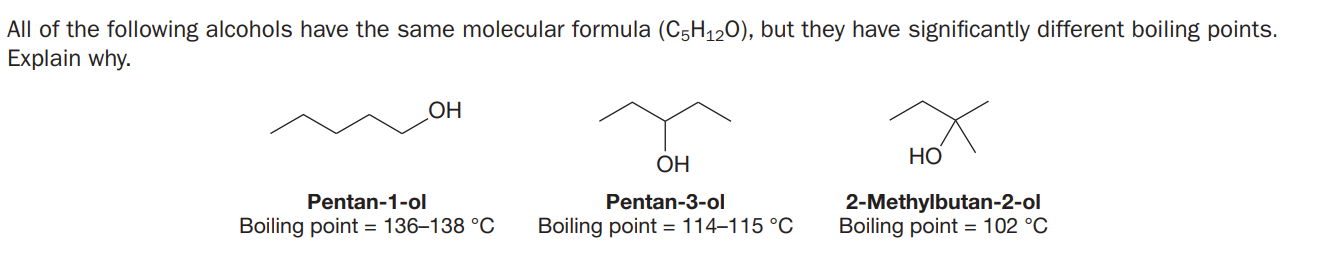
Transcribed Image Text:All of the following alcohols have the same molecular formula (C5H120), but they have significantly different boiling points.
Explain why.
OH
НО
ОН
2-Methylbutan-2-ol
Boiling point = 102 °C
Pentan-1-ol
Pentan-3-ol
Boiling point = 136–138 °C
Boiling point = 114–115 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning