alphatic? b. The N atom in adrenaline is 1°, 2°, 3°, 4°? c. How many p orbitals are use to make double bonds in adrenaline? d. Will adrenaline react as a nucleophile or electrophile in chemical reactions? но H CH2- NHCH3 A Adrenal
alphatic? b. The N atom in adrenaline is 1°, 2°, 3°, 4°? c. How many p orbitals are use to make double bonds in adrenaline? d. Will adrenaline react as a nucleophile or electrophile in chemical reactions? но H CH2- NHCH3 A Adrenal
Chapter24: Amines And Heterocycles
Section24.SE: Something Extra
Problem 75AP
Related questions
Question
100%
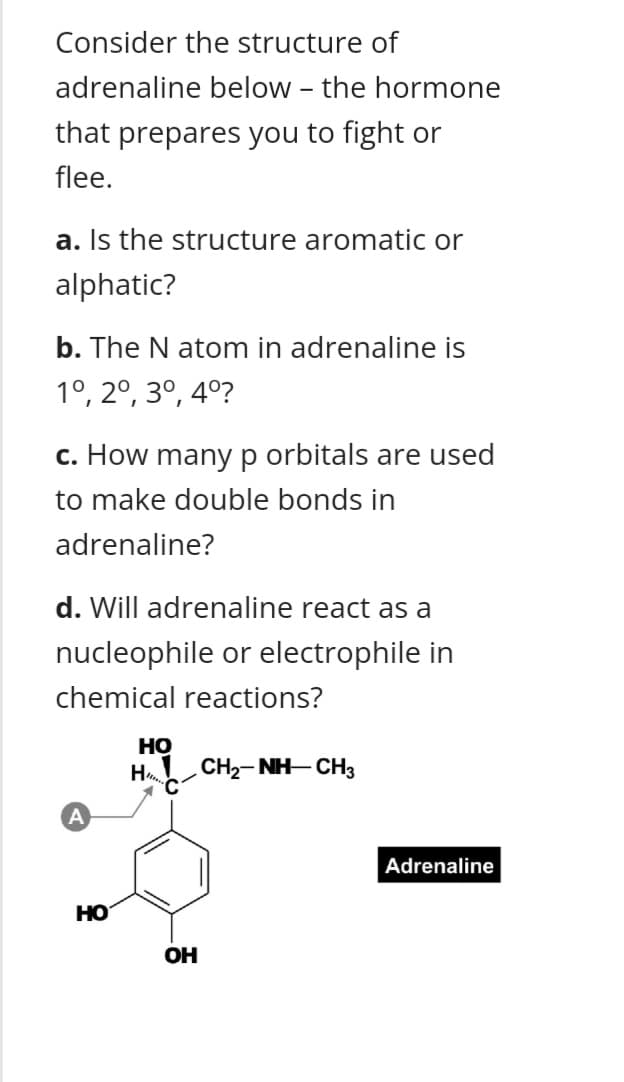
Transcribed Image Text:Consider the structure of
adrenaline below - the hormone
that prepares you to fight or
flee.
a. Is the structure aromatic or
alphatic?
b. The N atom in adrenaline is
1°, 2°, 3º, 4º?
c. How manyp orbitals are used
to make double bonds in
adrenaline?
d. Will adrenaline react as a
nucleophile or electrophile in
chemical reactions?
Но
H CH2- NH-CH3
Adrenaline
но
OH
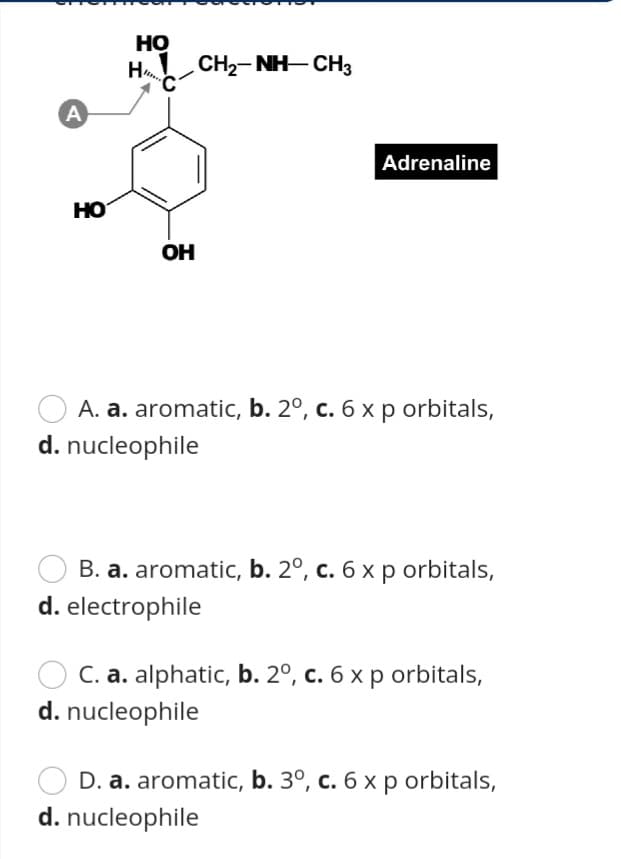
Transcribed Image Text:Но
H
CH2- NH- CH3
A
Adrenaline
HO
OH
A. a. aromatic, b. 2º, c. 6 x p orbitals,
d. nucleophile
B. a. aromatic, b. 2º, c. 6 x p orbitals,
d. electrophile
C. a. alphatic, b. 2º, c. 6 x p orbitals,
d. nucleophile
D. a. aromatic, b. 3º, c. 6 x p orbitals,
d. nucleophile
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning