If the pKa for formic acid is 3.75 the ratio of formate (A) to formic acid (HA) at pH 4.15 is 5:1. The pH of 1 liter of water to which is added 10 mL of 5.0 M HCI is 1.3. If an acid has a pka of 4.76 the Ka = 0.00001.
If the pKa for formic acid is 3.75 the ratio of formate (A) to formic acid (HA) at pH 4.15 is 5:1. The pH of 1 liter of water to which is added 10 mL of 5.0 M HCI is 1.3. If an acid has a pka of 4.76 the Ka = 0.00001.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section: Chapter Questions
Problem 4.33P: Complete the equation for the reaction between each Lewis acid-base pair. In each equation, label...
Related questions
Question
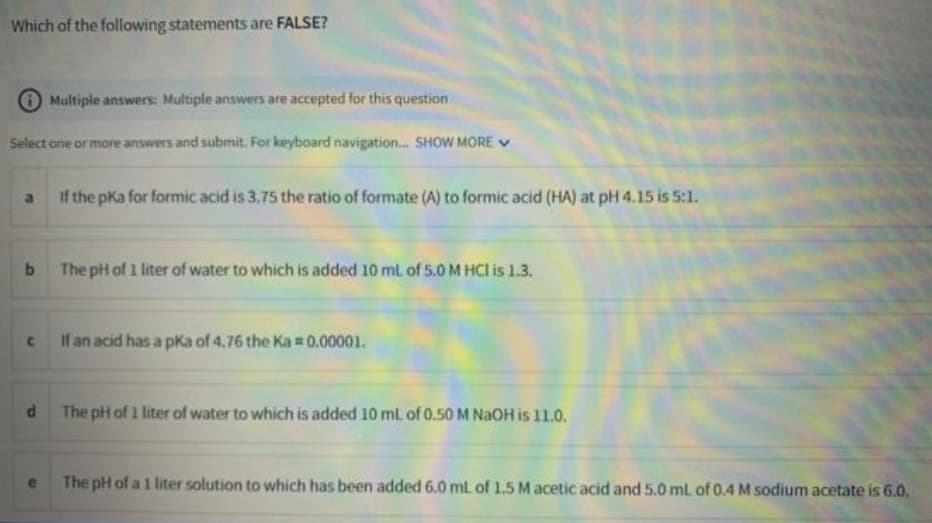
Transcribed Image Text:Which of the following statements are FALSE?
Multiple answers: Multiple answers are accepted for this question
Select one or more answers and submit. For keyboard navigation. SHOW MORE V
If the pKa for formic acid is 3.75 the ratio of formate (A) to formic acid (HA) at pH 4.15 is 5:1.
The pH of 1 liter of water to which is added 10 ml of 5.0 M HCl is 1.3.
If an acid has a pka of 4.76 the Ka =0.00001.
The pH of 1 liter of water to which is added 10 ml. of 0.50 M NAOH is 11.0.
The pH of a 1 liter solution to which has been added 6.0 mL of 1.5 M acetic acid and 5.0 ml of 0.4 M sodium acetate is 6.0.
to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
