Although cannabinol and methyl alcohol are both alcohols, cannabinol is very slightly soluble in methyl alcohol at room temperature. Explain. CH3 ОН CH3 CH3 CH2CH2CH,CH2CH3 Cannabinol
Although cannabinol and methyl alcohol are both alcohols, cannabinol is very slightly soluble in methyl alcohol at room temperature. Explain. CH3 ОН CH3 CH3 CH2CH2CH,CH2CH3 Cannabinol
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter13: Alcohols, Phenols, And Ethers
Section: Chapter Questions
Problem 13.72E
Related questions
Question
Please help and explain problem
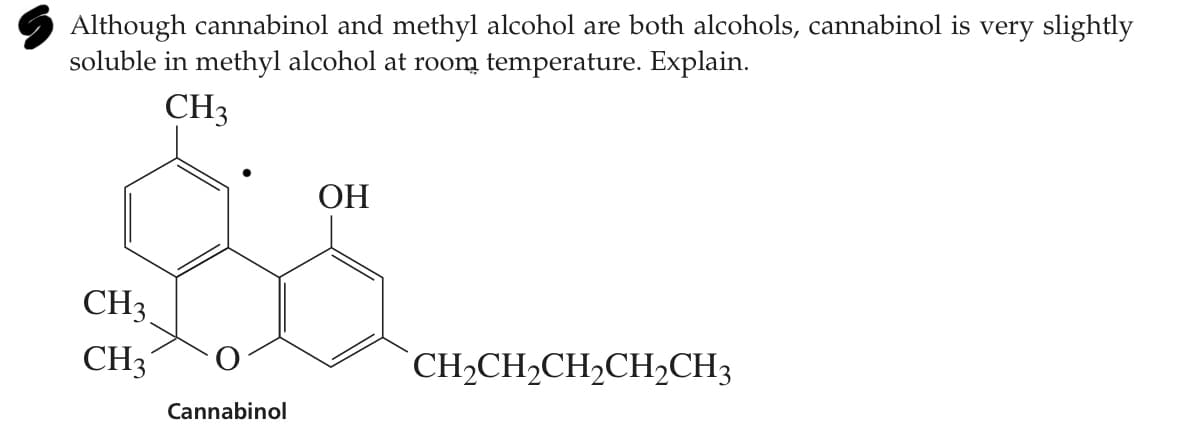
Transcribed Image Text:S Although cannabinol and methyl alcohol are both alcohols, cannabinol is very slightly
soluble in methyl alcohol at room temperature. Explain.
CH3
ОН
CH3
CH3
CH2CH,CH2CH2CH3
Cannabinol
Expert Solution
Step 1
Since polar solutes dissolve in polar solvents and non-polar solutes dissolve in non-polar solvents.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning