Aluminum (Al) reacts with chlorine (Cl,) gas to form aluminum chloride. Given the position of aluminum in the periodic table, what charge should 2 the aluminum ion have in this compound? A +4 -5 C +3 D +1
Aluminum (Al) reacts with chlorine (Cl,) gas to form aluminum chloride. Given the position of aluminum in the periodic table, what charge should 2 the aluminum ion have in this compound? A +4 -5 C +3 D +1
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter7: Ionic Compounds And Metals
Section: Chapter Questions
Problem 66A
Related questions
Question
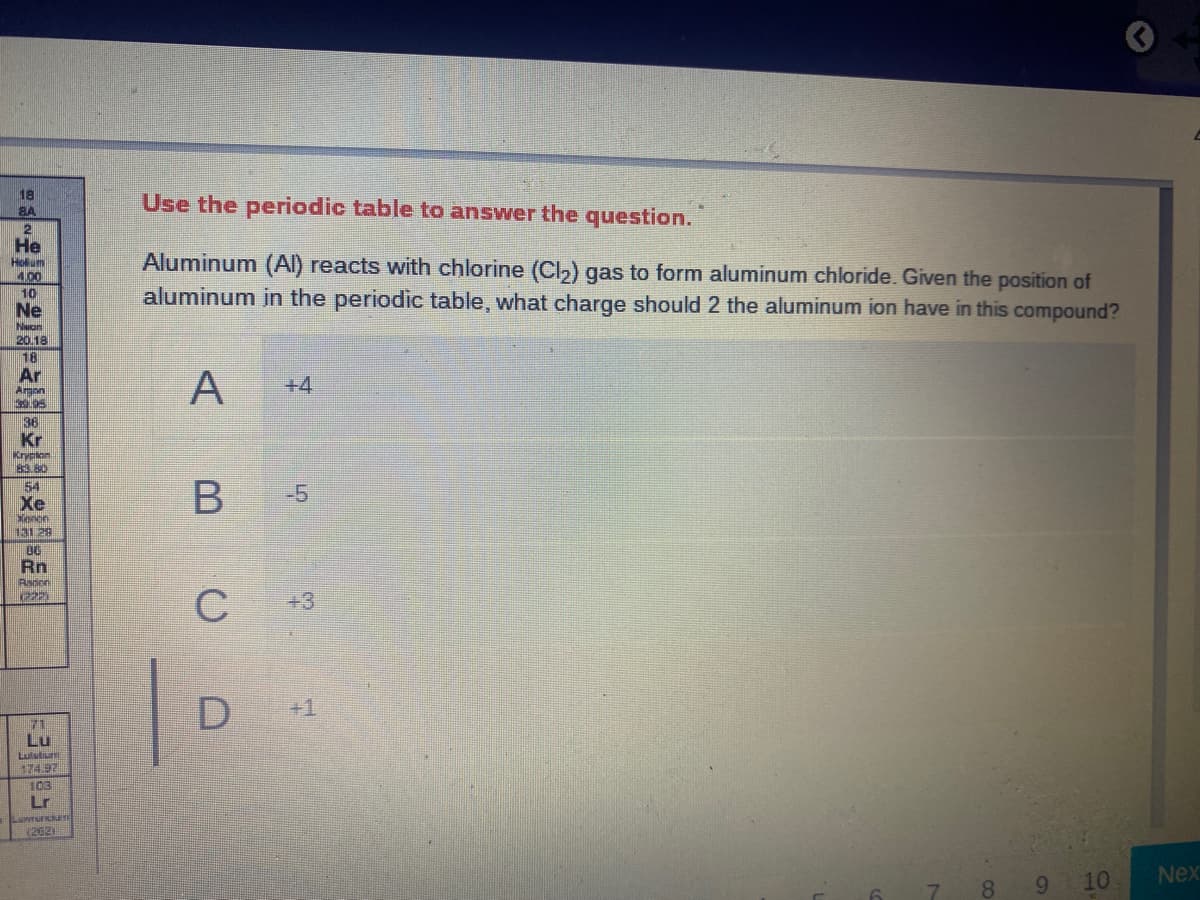
Transcribed Image Text:18
Use the periodic table to answer the question.
8A
He
Holum
4.00
Aluminum (Al) reacts with chlorine (Cl2) gas to form aluminum chloride. Given the position of
aluminum in the periodic table, what charge should 2 the aluminum ion have in this compound?
10
Ne
Nean
20.18
18
Ar
Argon
A
+4
36
Kr
Kryplon
3.80
54
Xe
Xonen
131 28
-5
Rn
C
+3
(222)
+1
71
Lu
Lulutiu
174.97
103
Lr
Luur
2621
10
Nex
8.
80
B
Transcribed Image Text:CHEMISTRY
Use the
Aluminu
Key
aluminur
11-
Na -Eemont symbol
Sodium- Element name
Atomic number
22.99
Average atomic mass*
A
anganer
Ir
192 25
105
MI
Db
Sg
Meitnan
(261)
Ce
Sm
Dy
Ho
Pr
*is number ion parantheses, then
t refors to the atomic mass of the
most stable isoope.
150 30
182 50
Th
Pa
Pu
Cf
Es
232.01 231.04
238 03
品 1p32
品 8あa 0
8>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning