Ammonia(NH3) can be produced by the reaction of hydrogen and nitrogen shown in the chemical equation below: N2+3H2→2NH3 If a student makes calculations and finds out that the theoretical yield of ammonia is 107.0g and the actual yield is 92.0g, what is the percent yield?
Ammonia(NH3) can be produced by the reaction of hydrogen and nitrogen shown in the chemical equation below: N2+3H2→2NH3 If a student makes calculations and finds out that the theoretical yield of ammonia is 107.0g and the actual yield is 92.0g, what is the percent yield?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter6: Chemical Calculations: Formula Masses, Moles, And Chemical Equations
Section: Chapter Questions
Problem 6.84EP: In an experiment designed to produce calcium oxide by the chemical reaction 2Ca + O2 2CaO 177.2 g...
Related questions
Question
Ammonia(NH3) can be produced by the reaction of hydrogen and nitrogen shown in the chemical equation below: N2+3H2→2NH3 If a student makes calculations and finds out that the theoretical yield of ammonia is 107.0g and the actual yield is 92.0g, what is the percent yield?
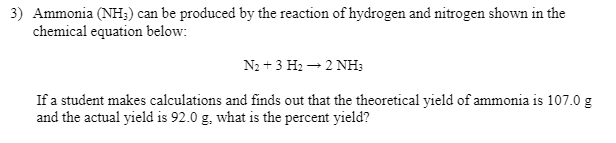
Transcribed Image Text:3) Ammonia (NH;) can be produced by the reaction of hydrogen and nitrogen shown in the
chemical equation below:
N2 + 3 H2 – 2 NH;
If a student makes calculations and finds out that the theoretical yield of ammonia is 107.0 g
and the actual yield is 92.0 g, what is the percent yield?
Expert Solution
Given
A numerical problem based on percent yield calculation, which is to be accomplished.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning