When heated with sulfuric or phosphoric acid, cyclohexanol, C6H110H, is converted to cyclohexene, C6H10. The balanced chemical equation for the reaction is shown below. C6H11OH(1) → C6H10(1) + H20(1) If the percent yield is 83%, what mass of cyclohexanol must we use to obtain 25 g of cyclohexene?
When heated with sulfuric or phosphoric acid, cyclohexanol, C6H110H, is converted to cyclohexene, C6H10. The balanced chemical equation for the reaction is shown below. C6H11OH(1) → C6H10(1) + H20(1) If the percent yield is 83%, what mass of cyclohexanol must we use to obtain 25 g of cyclohexene?
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 49A
Related questions
Question
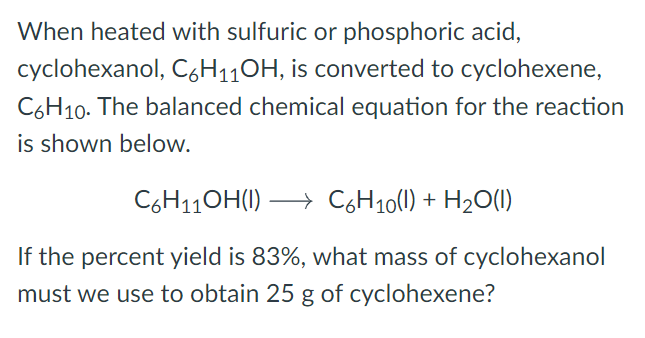
Transcribed Image Text:When heated with sulfuric or phosphoric acid,
cyclohexanol, C6H110H, is converted to cyclohexene,
C6H10. The balanced chemical equation for the reaction
is shown below.
C6H11OH(1)
→ C6H10(1) + H20(1)
If the percent yield is 83%, what mass of cyclohexanol
must we use to obtain 25 g of cyclohexene?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: Please show all steps clearly, than
Q: Write the Boolean equations for the circuits of Figure P6–7. Reduce the equations to their simplest…
Q: Kamrie wants to paint her living room. The rectangular room measures 20 feet by 15 feet and has…
Q: Fez wants to paint his basement family room. The room measures 40 feet by 25 feet and has 8-foot…
Q: In the past several years, Shakira had loaned money to Shakira Incorporated (an S corporation) to…
Q: What is the scope, purpose, and function of the Institutional Review Boards (IRB)
Q: Chandra was the sole shareholder of Pet Emporium, which was originally formed as an S corporation.…
Q: Steve is planning to paint his bathroom. The bathroom measures 8 feet by 9 feet with 8-foot…
Q: Be sure to answer all parts.
Calculate the equilibrium constants for decomposition of the following…
Q: What is the scope, purpose, and function of the Institutional Review Boards (IRB)
Q: Estimate the area of the region bounded by the graph of f(x)=x +4 and the x-axis on [0,6] in the…
Q: As we go along the electromagnetic spectrum starting at radio waves and ending on gamma rays, which…
Q: #8 Given
-
f(x) = 2x5 – 7x² +9x³ − 18x² +4x+40
Use the Rational Zeros Theorem to determine the…
Q: If light travels from air into flint glass, and the angle of refraction is 30°, what is the angle of…
Q: Green light can have a wavelength of 550 nm. What would be the frequency of this light?
A. 1.65…
Q: Photo
G
Find the surface area of the part of the plane z = 4+ 3x + 7y that lies
inside the cylinder…
Q: KHTK, the flagship station for the Sacramento Kings, transmits its broadcasts at a frequency of 1140…
Q: The following table lists all costs of quality incurred by Kate's shop last year.
Annual…
Q: Sarah recently sold her partnership interest for significantly more than her outside basis in the…
Q: Air
Perpendicular
Water
Incident
Ray
In the above diagram a light ray is about to leave water and…
Q: As we go along the electromagnetic spectrum starting at radio waves and ending on gamma rays, which…