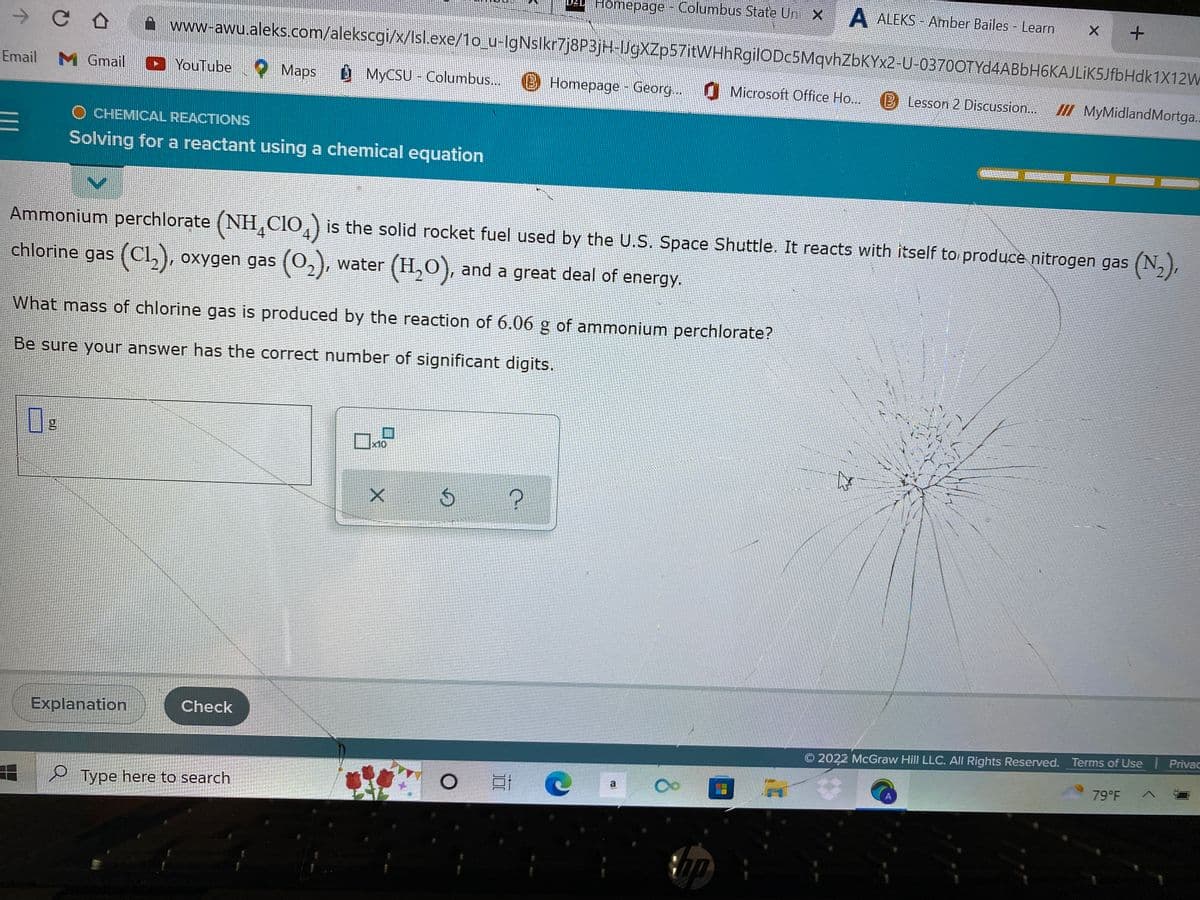
Ammonium perchlorate (NH CIO) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas chlorine gas (C1₂), oxygen gas (0₂), water (H₂O), and a great deal of energy. (N What mass of chlorine gas is produced by the reaction of 6.06 g of ammonium perchlorate? Be sure your answer has the correct number of significant digits. M S x10 X
Ammonium perchlorate (NH CIO) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas chlorine gas (C1₂), oxygen gas (0₂), water (H₂O), and a great deal of energy. (N What mass of chlorine gas is produced by the reaction of 6.06 g of ammonium perchlorate? Be sure your answer has the correct number of significant digits. M S x10 X
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.4QAP
Related questions
Question
Transcribed Image Text:D2 Homepage - Columbus State Uni X
A ALEKS-Amber Bailes - Learn
CO
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5MqvhZbKYx2-U-0370OTYd4ABbH6KAJLIK5JfbHdk1X12W
X +
Email M Gmail ►YouTube
Maps
MyCSU-Columbus...
Homepage - Georg... Microsoft Office Ho... 13 Lesson 2 Discussion...
III MyMidland Mortga..
O CHEMICAL REACTIONS
E
Solving for a reactant using a chemical equation
Ammonium perchlorate (NHÃCIO) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas (N₂),
chlorine gas (C1₂), oxygen gas (0₂), water (H₂O), and a great deal of energy.
What mass of chlorine gas is produced by the reaction of 6.06 g of ammonium perchlorate?
Be sure your answer has the correct number of significant digits.
g
x10
$
?
n
Explanation
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privac
O E
79°F
7
Check
Type here to search
X
a
Op
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

