Ammonium phosphate PO4) NH,),PO.) РО 3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 9.6 g of phosphoric acid? Round your answer to 2 significant digits.
Ammonium phosphate PO4) NH,),PO.) РО 3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia (NH,). What mass of ammonium phosphate is produced by the reaction of 9.6 g of phosphoric acid? Round your answer to 2 significant digits.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 25QAP: Cyclopropane mixed in the proper ratio with oxygen can be used as an anesthetic. At 755 mm Hg and...
Related questions
Question
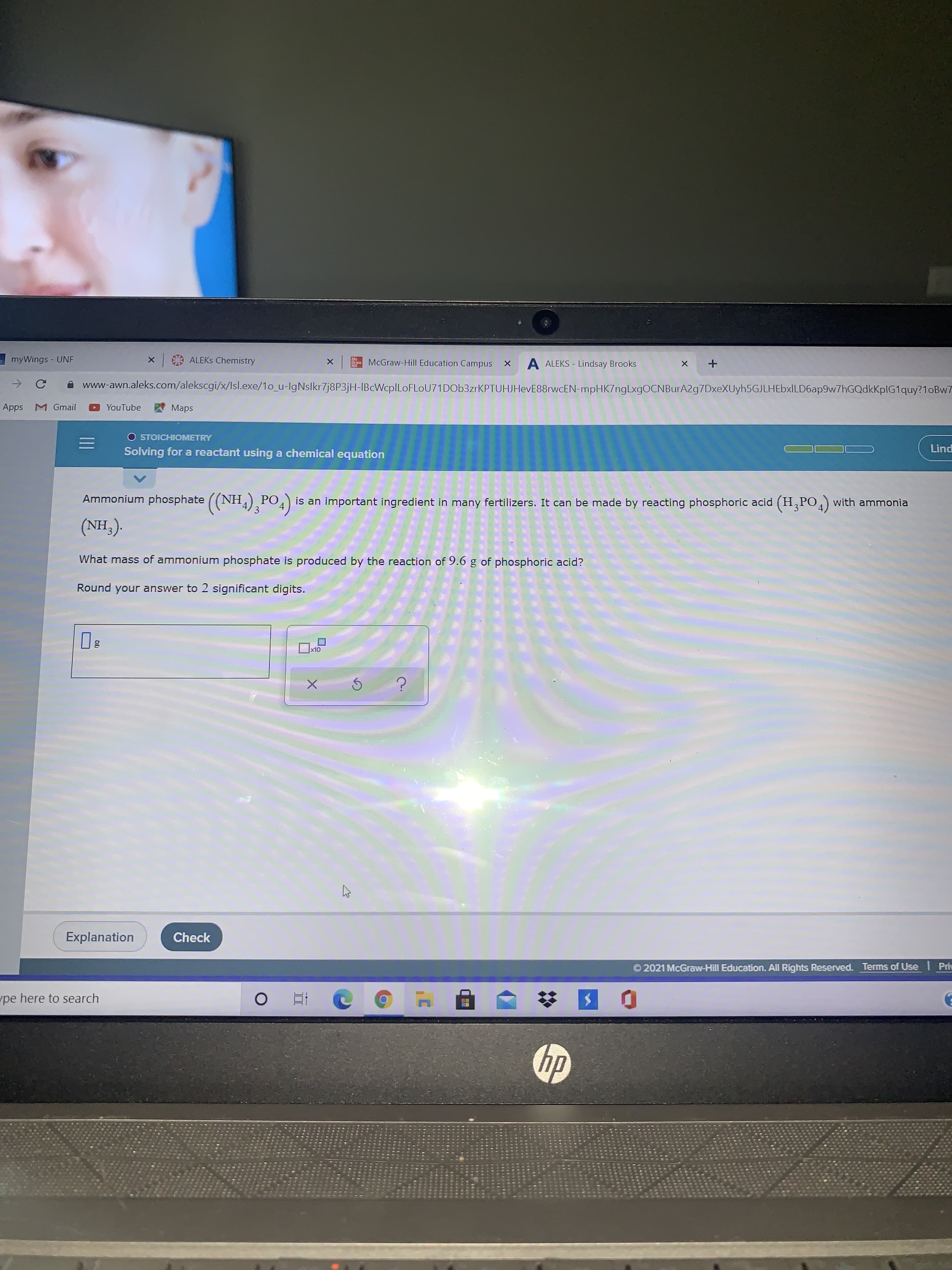
Transcribed Image Text:Ammonium phosphate PO4)
NH,),PO.)
РО
3
is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H,PO) with ammonia
(NH,).
What mass of ammonium phosphate is produced by the reaction of 9.6 g of phosphoric acid?
Round your answer to 2 significant digits.
Expert Solution
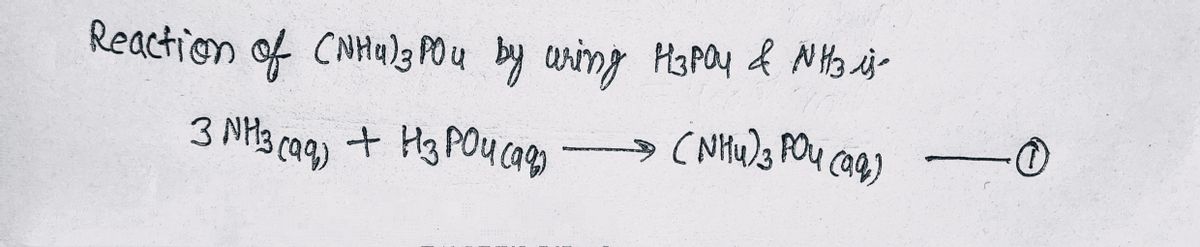
Step 1 Reaction
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning