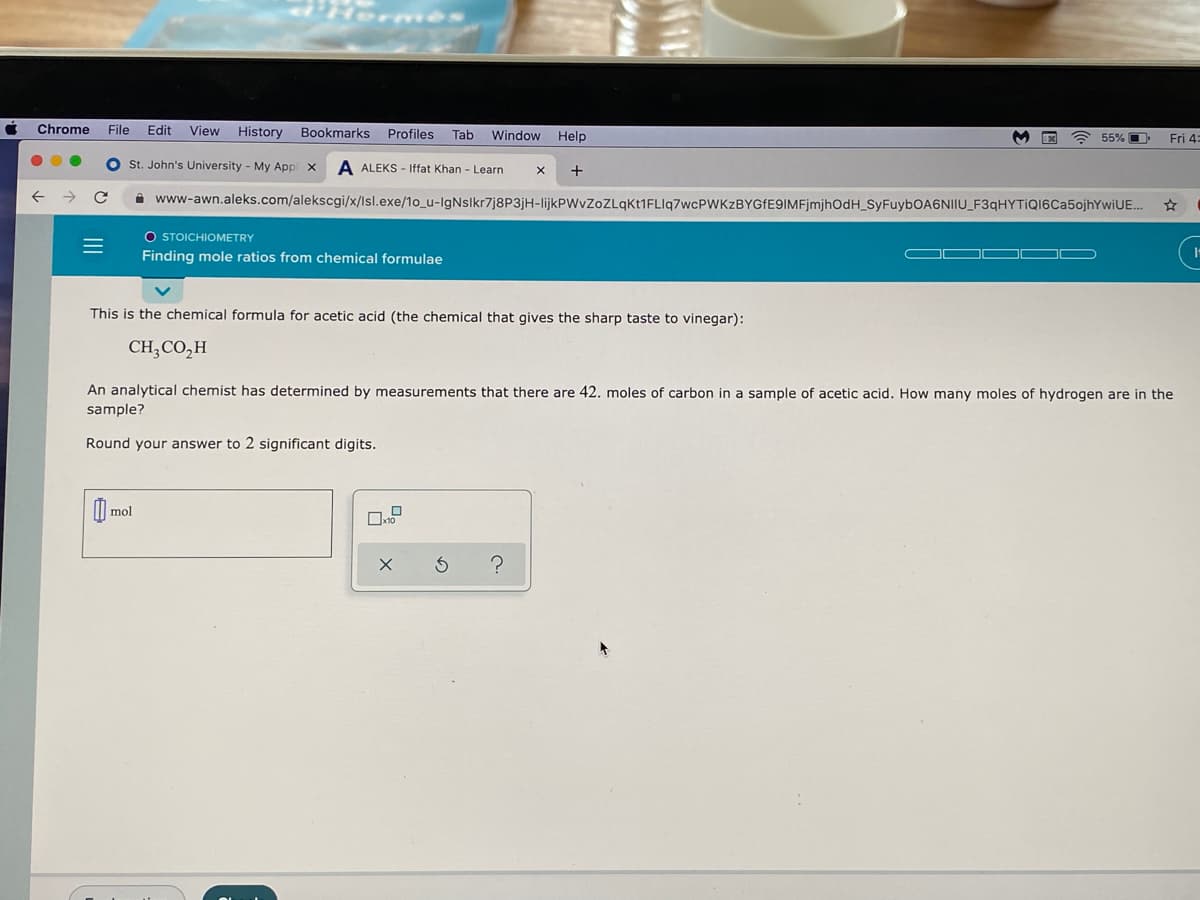
This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar): CH;CO,H An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are in the sample? Round your answer to 2 significant digits.
This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar): CH;CO,H An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are in the sample? Round your answer to 2 significant digits.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 16P: In the problem 15 above, what is vy , the y-component of the electron’s velocity, when it has...
Related questions
Question
Transcribed Image Text:Chrome
File
Edit
View History
Bookmarks Profiles
Window
M E ? 55% O
Tab
Help
Fri 4:
O st. John's University - My Appl x
A ALEKS - Iffat Khan - Learn
i www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjmjhOdH_SyFuybOA6NIIU_F3qHYTiQI6Ca5ojhYwiUE...
O STOICHIOMETRY
Finding mole ratios from chemical formulae
This is the chemical formula for acetic acid (the chemical that gives the sharp taste to vinegar):
CH,CO,H
An analytical chemist has determined by measurements that there are 42. moles of carbon in a sample of acetic acid. How many moles of hydrogen are in the
sample?
Round your answer to 2 significant digits.
mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

