Amount sed Compunds Pomule 25 1049 (166€ |-in.ge Acehe Acid IsOamyl Alcchel kom) Conarkold Jelkanc ccdS 4ml Jodium bucarbonole ) Goml Jodum Chlonde 20 ml 33.15 SCity Oz 98-08 (H2 SC4 24.007 NGHCO3 ) 2.20g ( 50°C 1.3g 10 ml 52 44 (NOCI 2.17 384 C 142-64 Julfae I samyl Acdale Calcutatuns Acehe aud 20l x l.099= 20.84 rams of acetie acid ISOomyl Mcohol 6ml x bi8139 = 13.0 grum of isoamyl Afcohl %3D 20,3 of gams A Cehe acud I mol of CaltDz = 013463 moles 'C2H202. Acch aud I mol of ChaoyOz = 0:1414 moles CTH1Y02. soamyl Mechon 아 Isoamyl Mculel 38.15g
Amount sed Compunds Pomule 25 1049 (166€ |-in.ge Acehe Acid IsOamyl Alcchel kom) Conarkold Jelkanc ccdS 4ml Jodium bucarbonole ) Goml Jodum Chlonde 20 ml 33.15 SCity Oz 98-08 (H2 SC4 24.007 NGHCO3 ) 2.20g ( 50°C 1.3g 10 ml 52 44 (NOCI 2.17 384 C 142-64 Julfae I samyl Acdale Calcutatuns Acehe aud 20l x l.099= 20.84 rams of acetie acid ISOomyl Mcohol 6ml x bi8139 = 13.0 grum of isoamyl Afcohl %3D 20,3 of gams A Cehe acud I mol of CaltDz = 013463 moles 'C2H202. Acch aud I mol of ChaoyOz = 0:1414 moles CTH1Y02. soamyl Mechon 아 Isoamyl Mculel 38.15g
Chapter5: Equilibrium, Activity And Solving Equations
Section: Chapter Questions
Problem 4P
Related questions
Question
using this info . how can i determine the theoretical yield
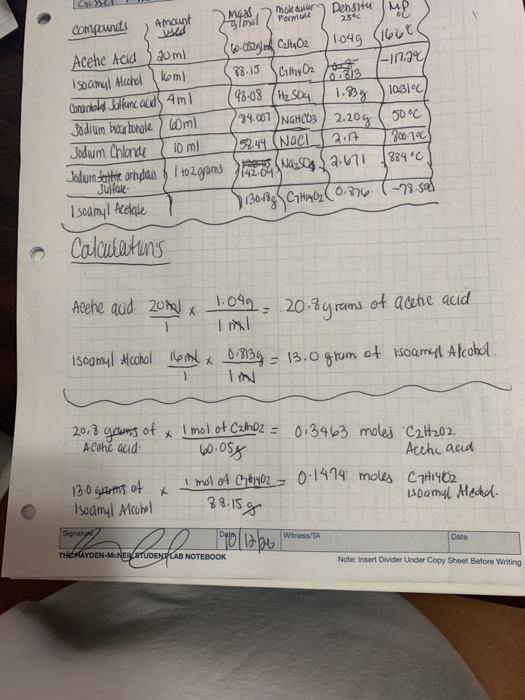
Transcribed Image Text:mok dular
Pomule
Densitu Me
Compeunds
Amount
25
104g (I6して
|-in.ge
Acehe Acid
Isoamyl Alcahel koml
Conankoled Jalfanc acidS 4ml
Jodium btarbonole ) Dml
Jodum Chlonde
Jolum tethie orhdan I t02grams
20 ml
38-15 Ciy O2
98-08 (H2 SO4
34.007 )NGHC03 ) 2.20g ( 50°C
0.813
1.8g ) la31°c
10 ml
52 44 (Noel
2.17
B00-79C
884 °C
Julfale-
-79-sad
I seamyl Acelale
Calculatuns
Acehe aud 20hal *
1.099=
20-3y rams
of acetie acid
ISoamyl Hcohol lom x b8134= 13.0 grum
of soamyl Alcohd .
%3D
20.3 gams
I mol ot CatDZ = 0:3463 moles "C2H202.
60.05g
I mol of GydoyOz = 0:1474 moles C THI402
38.15g
of
A Cehc aud
Acchc aud
130 Groms of
Isoamyl Alcubal
soamul Alechd.
Signatiyre
Dete
Witness/TA
Date
THEAYDEN-MCNESTUDENTLAB NOTEBOOK
Note: Insert Divider Under Copy Sheet Before Writing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you





