Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.3: Stability Of Cycloalkanes: Ring Strain
Problem 8P: Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are...
Related questions
Question
Calculate strain energy for the conformer pictured below, using strain energy increments from the table.
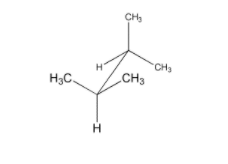
Transcribed Image Text:CH,
CH3
CH3
H3C
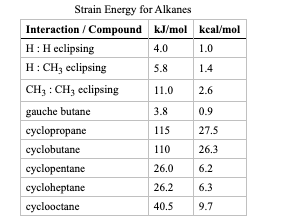
Transcribed Image Text:Strain Energy for Alkanes
Interaction / Compound kJ/mol kcal/mol
H:H eclipsing
H: CH, eclipsing
4.0
1.0
5.8
1.4
CH3 : CH3 eclipsing
11.0
2.6
gauche butane
3.8
0.9
cyclopropane
115
27.5
cyclobutane
110
26.3
cyclopentane
26.0
6.2
cycloheptane
26.2
6.3
cyclooctane
40.5
9.7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning