An alpha particle (a : = two protons, two neutrons) moves into a stationary gold atom (Au = 79 protons, 118 neutrons), passing through the electron region that surrounds the gold nucleus like a shell and headed directly toward the nucleus (see below). The alpha particle slows until it momentarily stops when its center is at radial distance r = 9.23 fm from the nuclear center. Then it moves back along its incoming path. (Because the gold nucleus is much more massive than the alpha particle, we can assume the gold nucleus does not move.) What was the kinetic energy K; of the alpha particle when it was initially far away (hence external to the gold atom)? Assume that the only force acting between the alpha particle and the gold nucleus is the (electrostatic) Coulomb force and treat each as a single charged particle.
An alpha particle (a : = two protons, two neutrons) moves into a stationary gold atom (Au = 79 protons, 118 neutrons), passing through the electron region that surrounds the gold nucleus like a shell and headed directly toward the nucleus (see below). The alpha particle slows until it momentarily stops when its center is at radial distance r = 9.23 fm from the nuclear center. Then it moves back along its incoming path. (Because the gold nucleus is much more massive than the alpha particle, we can assume the gold nucleus does not move.) What was the kinetic energy K; of the alpha particle when it was initially far away (hence external to the gold atom)? Assume that the only force acting between the alpha particle and the gold nucleus is the (electrostatic) Coulomb force and treat each as a single charged particle.
Chapter5: Electric Charges And Fields
Section: Chapter Questions
Problem 120AP: A particle of charge q and mass m is placed at the center of a uniformly charged ring of total...
Related questions
Question
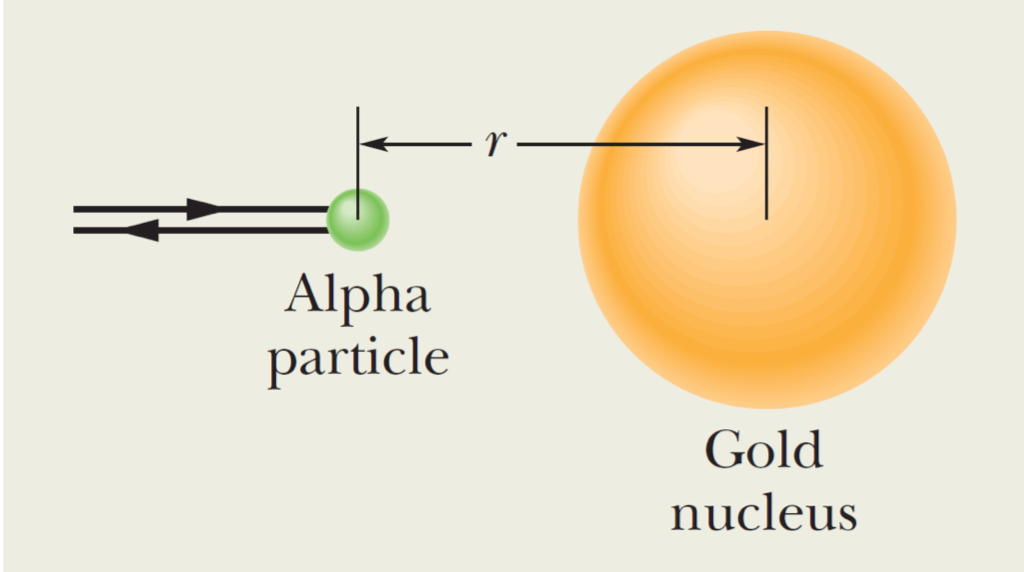
Transcribed Image Text:Alpha
particle
Gold
nucleus
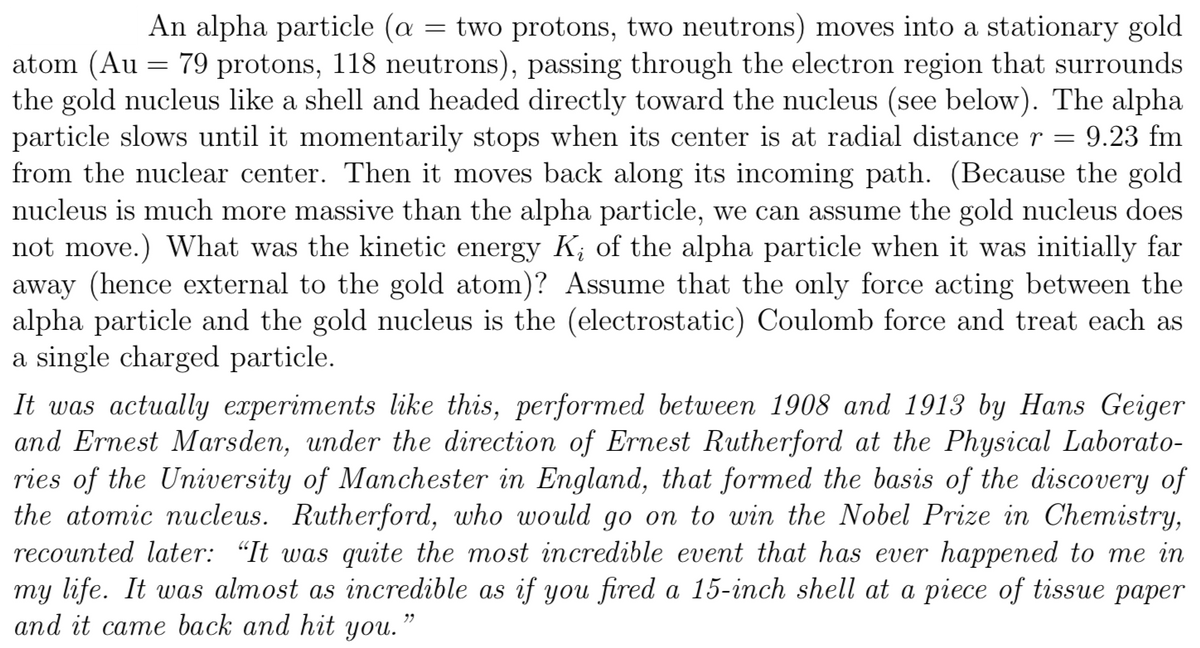
Transcribed Image Text:An alpha particle (a
two protons, two neutrons) moves into a stationary gold
atom (Au = 79 protons, 118 neutrons), passing through the electron region that surrounds
the gold nucleus like a shell and headed directly toward the nucleus (see below). The alpha
particle slows until it momentarily stops when its center is at radial distance r
from the nuclear center. Then it moves back along its incoming path. (Because the gold
nucleus is much more massive than the alpha particle, we can assume the gold nucleus does
not move.) What was the kinetic energy K; of the alpha particle when it was initially far
away (hence external to the gold atom)? Assume that the only force acting between the
alpha particle and the gold nucleus is the (electrostatic) Coulomb force and treat each as
a single charged particle.
9.23 fm
It was actually experiments like this, performed between 1908 and 1913 by Hans Geiger
and Ernest Marsden, under the direction of Ernest Rutherford at the Physical Laborato-
ries of the University of Manchester in England, that formed the basis of the discovery of
the atomic nucleus. Rutherford, who would go on to win the Nobel Prize in Chemistry,
recounted later: “It was quite the most incredible event that has ever happened to me in
my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper
and it came back and hit you.’
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning