An aqueous solution is prepared to be 0.408 M in sodium formate and 0.291 M in acetic acid. (1) Is this solution a buffer solution? (2) What is the pH of this solution? pH = (3) If 0.128 moles of hydrobromic acid are added to one liter of this solution, what is the pH of the resulting solution? pH = Use the Tables link in the References for any equilibrium constants that are required.
An aqueous solution is prepared to be 0.408 M in sodium formate and 0.291 M in acetic acid. (1) Is this solution a buffer solution? (2) What is the pH of this solution? pH = (3) If 0.128 moles of hydrobromic acid are added to one liter of this solution, what is the pH of the resulting solution? pH = Use the Tables link in the References for any equilibrium constants that are required.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 46P: Sulfanilic acid (NH2C6H4SO3H) is used in manufacturing dyes. It ionizes in water according to the...
Related questions
Question
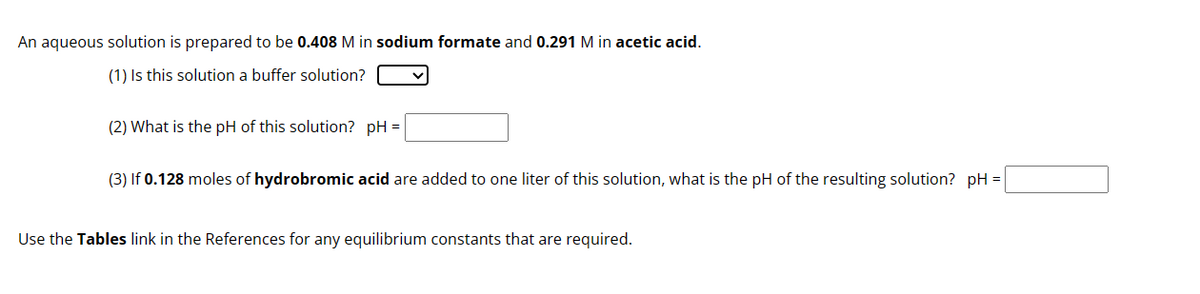
Transcribed Image Text:An aqueous solution is prepared to be 0.408 M in sodium formate and 0.291 M in acetic acid.
(1) Is this solution a buffer solution?
(2) What is the pH of this solution? pH =
(3) If 0.128 moles of hydrobromic acid are added to one liter of this solution, what is the pH of the resulting solution? pH =
Use the Tables link in the References for any equilibrium constants that are required.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning