An atom has a mass number of 30 and 16 neutrons. What is the atomic number of this aton Express your answer as an integer. > View Available Higs) Ηνα ΑΣφ atomic number =
An atom has a mass number of 30 and 16 neutrons. What is the atomic number of this aton Express your answer as an integer. > View Available Higs) Ηνα ΑΣφ atomic number =
Chapter1: Excel Basics
Section: Chapter Questions
Problem 4P
Related questions
Question
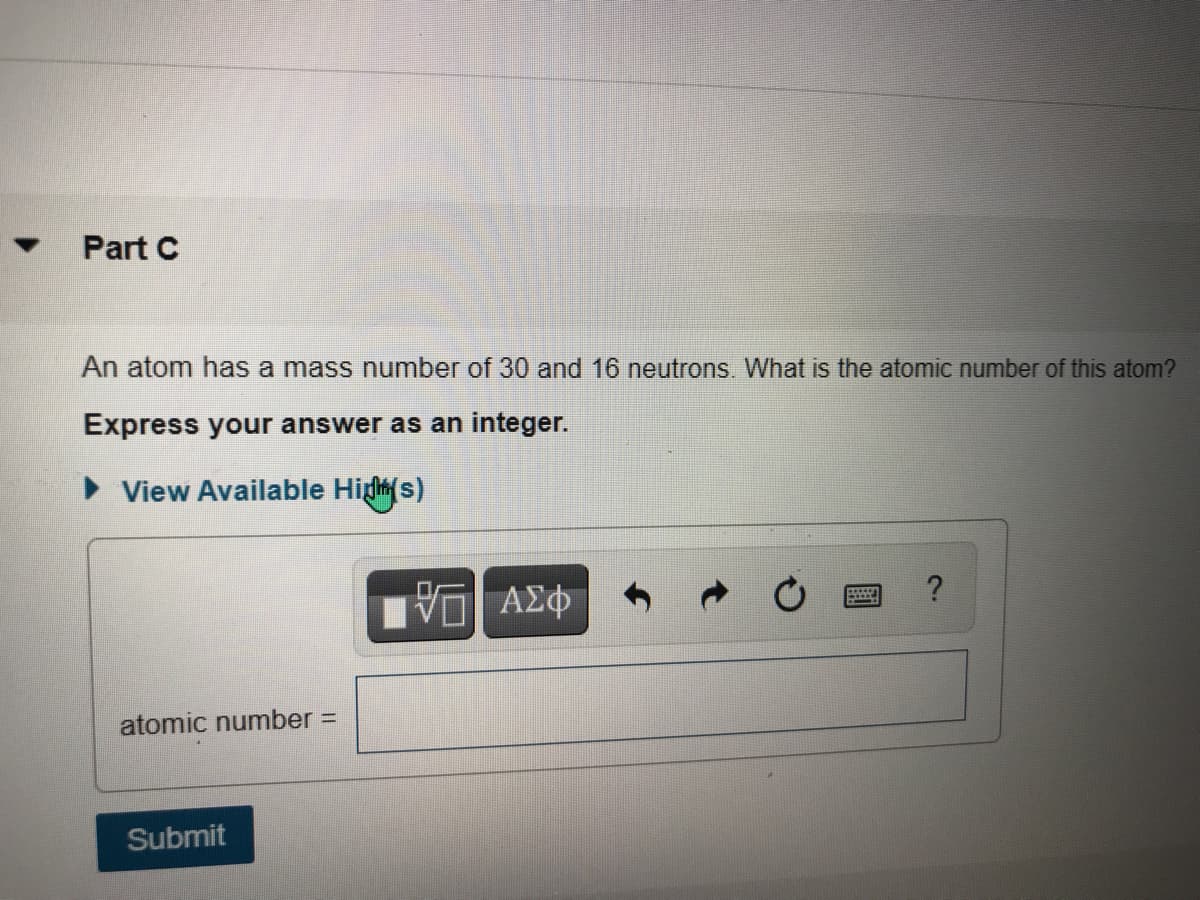
Transcribed Image Text:Part C
An atom has a mass number of 30 and 16 neutrons. What is the atomic number of this atom?
Express your answer as an integer.
• View Available Higs)
ΑΣΦ
atomic number =
Submit
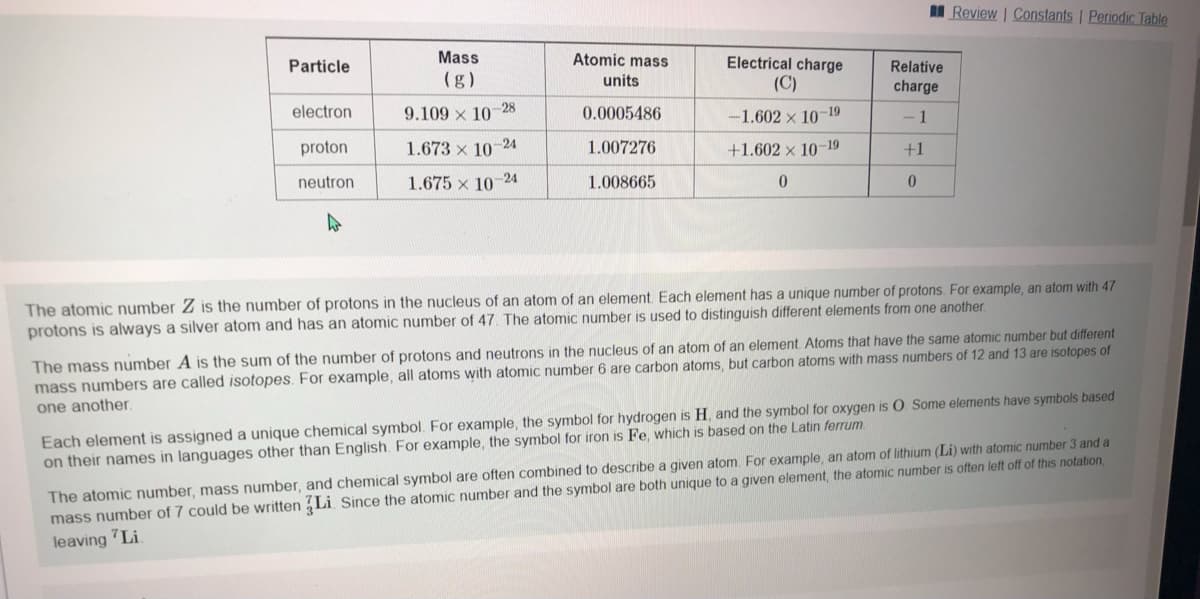
Transcribed Image Text:I Review | Constants | Periodic Table
Particle
Mass
Atomic mass
Electrical charge
Relative
(g)
units
(C)
charge
electron
9.109 x 10-28
0.0005486
-19
-1.602 x 10
- 1
proton
1.673 x 10
24
1.007276
+1.602 × 10-19
+1
neutron
1.675 x 10-24
1.008665
The atomic number Z is the number of protons in the nucleus of an atom of an element Each element has a unique number of protons. For example, an atom with 47
protons is always a silver atom and has an atomic number of 47. The atomic number is used to distinguish different elements from one another.
The mass number A is the sum of the number of protons and neutrons in the nucleus of an atom of an element. Atoms that have the same atomic number but different
mass numbers are called isotopes. For example, all atoms with atomic number 6 are carbon atoms, but carbon atoms with mass numbers of 12 and 13 are isotopes of
one another.
Each element is assigned a unique chemical symbol. For example, the symbol for hydrogen is H, and the symbol for oxygen is O. Some elements have symbols based
their names in languages other than English. For example, the symbol for iron is Fe, which is based on the Latin ferrum.
The atomic number, mass number, and chemical symbol are often combined to describe a given atom. For example, an atom of lithium (Li) with atomic number 3 and a
mass number of 7 could be written Li Since the atomic number and the symbol are both unique to a given element, the atomic number is often left off of this notation,
leaving Li.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

