An Expanding Monatomic Gas We start with 5.00 moles of an ideal monatomic gas with an initial temperature of 134 °C. The gas expands and, in the process, absorbs an amount of heat equal to 1280 J and does an amount of work equal to 2000J. Part A What is the final temperature Tinal of the gas? Use R = 8.3145 J/(mol · K) for the ideal gas constant. • View Available Hint(s) DA ΑΣφ ? Tinal = °C Submit Request Answer
An Expanding Monatomic Gas We start with 5.00 moles of an ideal monatomic gas with an initial temperature of 134 °C. The gas expands and, in the process, absorbs an amount of heat equal to 1280 J and does an amount of work equal to 2000J. Part A What is the final temperature Tinal of the gas? Use R = 8.3145 J/(mol · K) for the ideal gas constant. • View Available Hint(s) DA ΑΣφ ? Tinal = °C Submit Request Answer
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 78P: Two moles of a monatomic ideal gas such as helium is compressed adiabatically and reversibly from a...
Related questions
Question
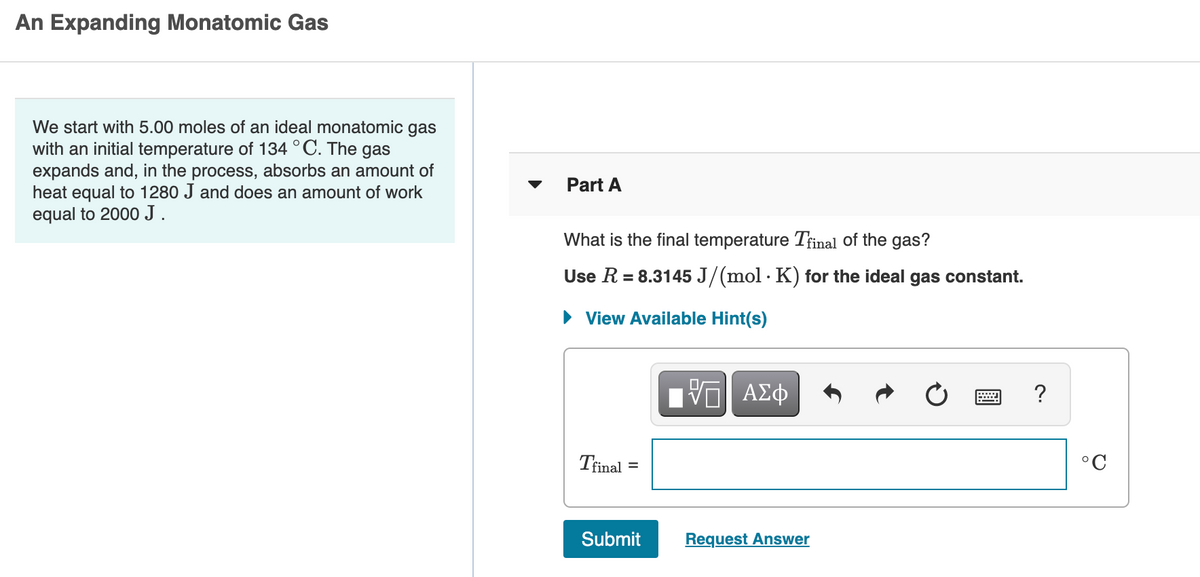
Transcribed Image Text:An Expanding Monatomic Gas
We start with 5.00 moles of an ideal monatomic gas
with an initial temperature of 134 °C. The gas
expands and, in the process, absorbs an amount of
heat equal to 1280 J and does an amount of work
Part A
equal to 2000J.
What is the final temperature Tfinal of the gas?
Use R = 8.3145 J/(mol · K) for the ideal gas constant.
View Available Hint(s)
ΠΥΠ ΑΣφ
?
Trinal
°C
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

