Anna Divi, a young and ambitious researcher, is planning to put up her very own research laboratory. However, she does not have the funds for this to happen. She then approached Alan Read, CEO of a well-known funding agency, to help her pursue her dream laboratory. Before Alan considers Anna's proposal, Anna must perform an experiment on determining the melting point of solutions. She must have an accurate result with a percent error not greater than 5%. The data on the experiment is given below. Help Anna achieve her goals by performing the necessary calculations below. Table 1. Data on the determination of the melting point of the solution. Parameters Values Theoretical freezing point of solvent, °C 54.6 Freezing point depression constant (k) of solvent, °C m1 3.21 mass of solute, g 0.100 mass of solvent, g 1.200 molar mass of solute, g mol1 116.30
Anna Divi, a young and ambitious researcher, is planning to put up her very own research laboratory. However, she does not have the funds for this to happen. She then approached Alan Read, CEO of a well-known funding agency, to help her pursue her dream laboratory. Before Alan considers Anna's proposal, Anna must perform an experiment on determining the melting point of solutions. She must have an accurate result with a percent error not greater than 5%. The data on the experiment is given below. Help Anna achieve her goals by performing the necessary calculations below. Table 1. Data on the determination of the melting point of the solution. Parameters Values Theoretical freezing point of solvent, °C 54.6 Freezing point depression constant (k) of solvent, °C m1 3.21 mass of solute, g 0.100 mass of solvent, g 1.200 molar mass of solute, g mol1 116.30
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.1: Units Of Concentration
Problem 1CYU: (a) If you dissolve 10.0 g (about one heaping teaspoonful) of sugar (sucrose, C12H22O11) in a cup of...
Related questions
Question
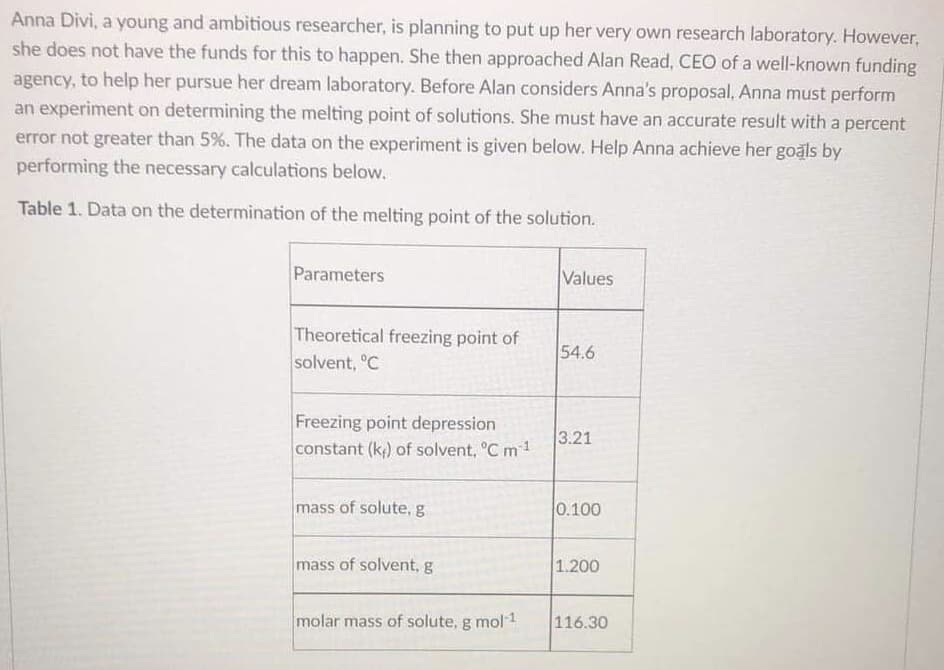
Transcribed Image Text:Anna Divi, a young and ambitious researcher, is planning to put up her very own research laboratory. However,
she does not have the funds for this to happen. She then approached Alan Read, CEO of a well-known funding
agency, to help her pursue her dream laboratory. Before Alan considers Anna's proposal, Anna must perform
an experiment on determining the melting point of solutions. She must have an accurate result with a percent
error not greater than 5%. The data on the experiment is given below. Help Anna achieve her goals by
performing the necessary calculations below.
Table 1. Data on the determination of the melting point of the solution.
Parameters
Values
Theoretical freezing point of
solvent, °C
54.6
Freezing point depression
constant (k;) of solvent, °C m1
3.21
mass of solute, g
0.100
mass of solvent, g
1.200
molar mass of solute, g mol 1
116.30
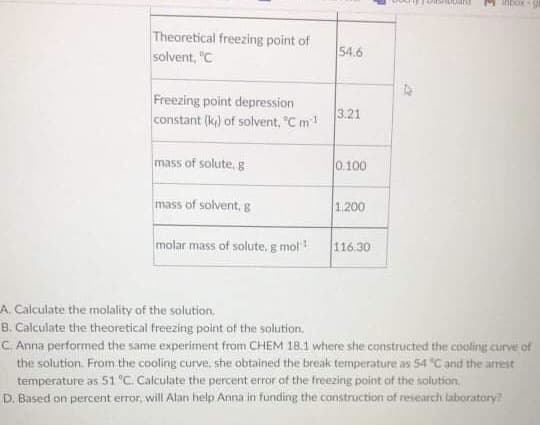
Transcribed Image Text:M Inbox
Theoretical freezing point of
solvent, "C
54.6
Freezing point depression
3.21
constant (k) of solvent, "C m1
mass of solute, g
0.100
mass of solvent, g
1.200
molar mass of solute, g mol
116.30
A. Calculate the molality of the solution.
B. Calculate the theoretical freezing point of the solution.
C Anna performed the same experiment from CHEM 18.1 where she constructed the cooling curve of
the solution. From the cooling curve, she obtained the break temperature as 54 "C and the arrest
temperature as 51 C Calculate the percent error of the freezing point of the solution,
D. Based on percent error, will Alan help Anna in funding the construction of research laboratory?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning