Another reagent that can be used to convert carboxylic acids into acid chlorides is phosphorus trichloride (PCI3), as shown in the scheme below. :0: :ö: :0: PCI2 + -PCI2 :0: + НОРC2 The mechanism, for the reaction, is shown above but the curved arrows and the formal charges, on the respective atoms, have been omitted. (a) Draw the missing curved arrows for each step (b) Supply any missing formal charges
Another reagent that can be used to convert carboxylic acids into acid chlorides is phosphorus trichloride (PCI3), as shown in the scheme below. :0: :ö: :0: PCI2 + -PCI2 :0: + НОРC2 The mechanism, for the reaction, is shown above but the curved arrows and the formal charges, on the respective atoms, have been omitted. (a) Draw the missing curved arrows for each step (b) Supply any missing formal charges
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 64AP: The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold...
Related questions
Question
Solve a and b with detailed explanation
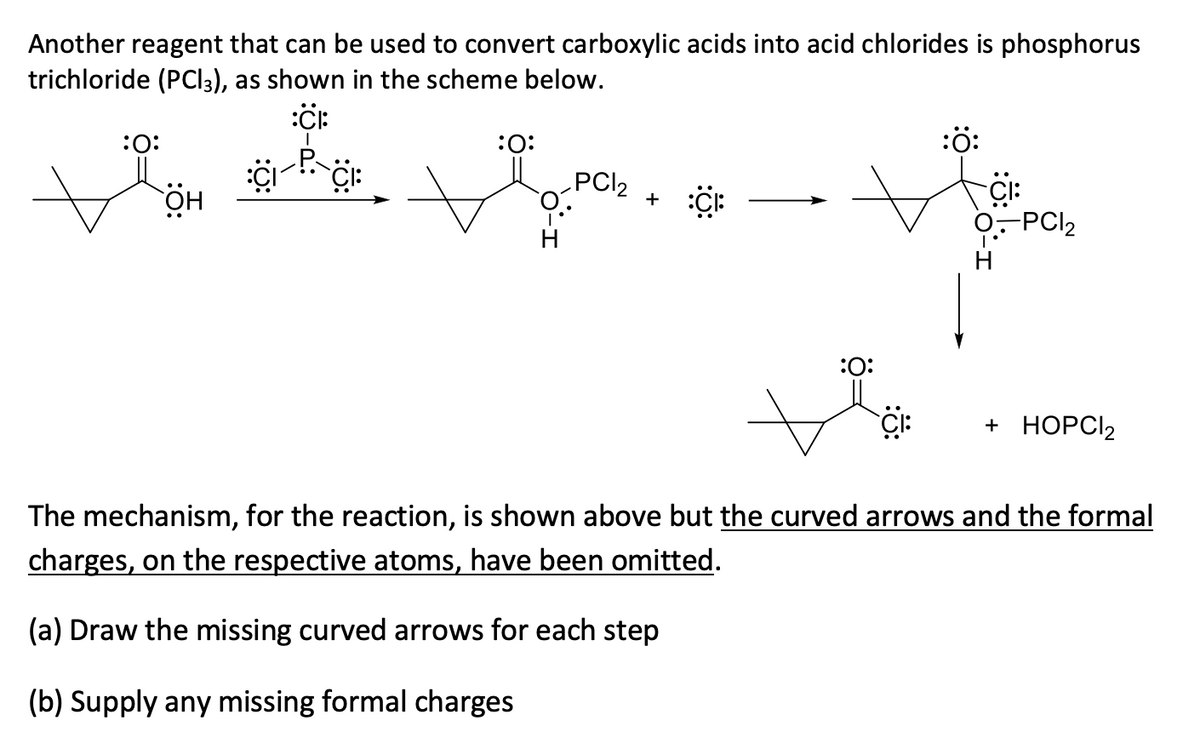
Transcribed Image Text:Another reagent that can be used to convert carboxylic acids into acid chlorides is phosphorus
trichloride (PCI3), as shown in the scheme below.
:ö:
:0:
PCI2
:O:
+
-PCI2
:0:
+ НОРC2
The mechanism, for the reaction, is shown above but the curved arrows and the formal
charges, on the respective atoms, have been omitted.
(a) Draw the missing curved arrows for each step
(b) Supply any missing formal charges
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning