The three Lews stnictures below are incomplete in that the non-bonding electrons are missing What is the TOTAL nurnter non-bonding pairs c! electrons that are missıng from the three structures? (Add the number of missing electron pairs on nitromethare to the number missing on the methylamide anion to the number missing on the azde anion) All formal charges are included. H CH3 N N N nitromethane methylamide azide anion
The three Lews stnictures below are incomplete in that the non-bonding electrons are missing What is the TOTAL nurnter non-bonding pairs c! electrons that are missıng from the three structures? (Add the number of missing electron pairs on nitromethare to the number missing on the methylamide anion to the number missing on the azde anion) All formal charges are included. H CH3 N N N nitromethane methylamide azide anion
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 19CTQ: The C=O double bond is called a “carbonyl bond.” Acetone and othercarbonyl compounds are introduced...
Related questions
Question
100%
!
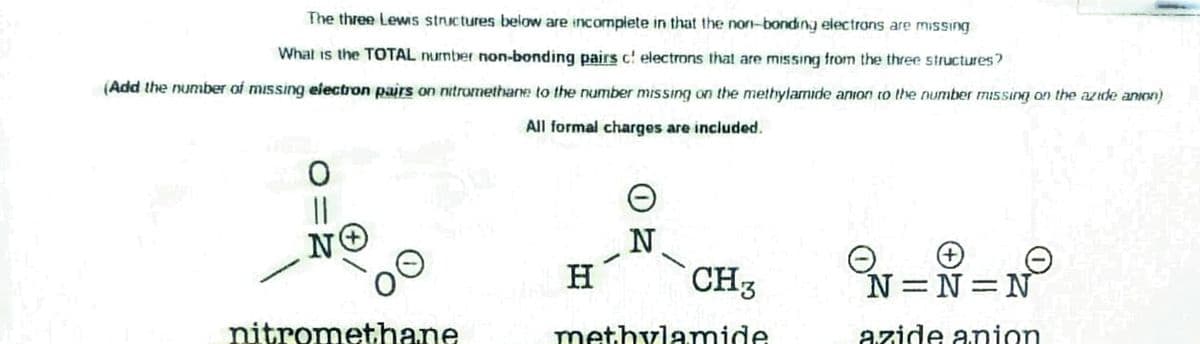
Transcribed Image Text:The three Lewis stnictures below are incomplete in that the non-bonding electrans are missing
What is the TOTAL nurnber non-bonding pairs c! electrons that are missing from the three structures?
(Add the number of missing electron pairs on nitromethane to the number missing on the methylamide anion to the number missing on the azide anion)
All formal charges are included.
||
N
H
CH3
N=N N
nitromethane
methylAmide
azide anion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning