Answer the questions posed with reference to the reaction profile shown. В D A E С 1. What letter(s) represent the starting material(s)? 2. What letter(s) represent the product(s)? 3. What letter(s) represent the intermediate(s)? 4. What letter(s) represent the transition state(s)? 5. How many steps are there in this reaction? 6. How many Es are there in this reaction? 7. If there is more than one step in this reaction, which is the rate-determining (slow) step? 8. Write the equation for this reaction: 9. Write the general form of the rate law for this reaction: 10. Which factor, if any, in the rate law changes if temperature is changed? Answer the following questions using the data provided here:
Answer the questions posed with reference to the reaction profile shown. В D A E С 1. What letter(s) represent the starting material(s)? 2. What letter(s) represent the product(s)? 3. What letter(s) represent the intermediate(s)? 4. What letter(s) represent the transition state(s)? 5. How many steps are there in this reaction? 6. How many Es are there in this reaction? 7. If there is more than one step in this reaction, which is the rate-determining (slow) step? 8. Write the equation for this reaction: 9. Write the general form of the rate law for this reaction: 10. Which factor, if any, in the rate law changes if temperature is changed? Answer the following questions using the data provided here:
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 18CTQ
Related questions
Question
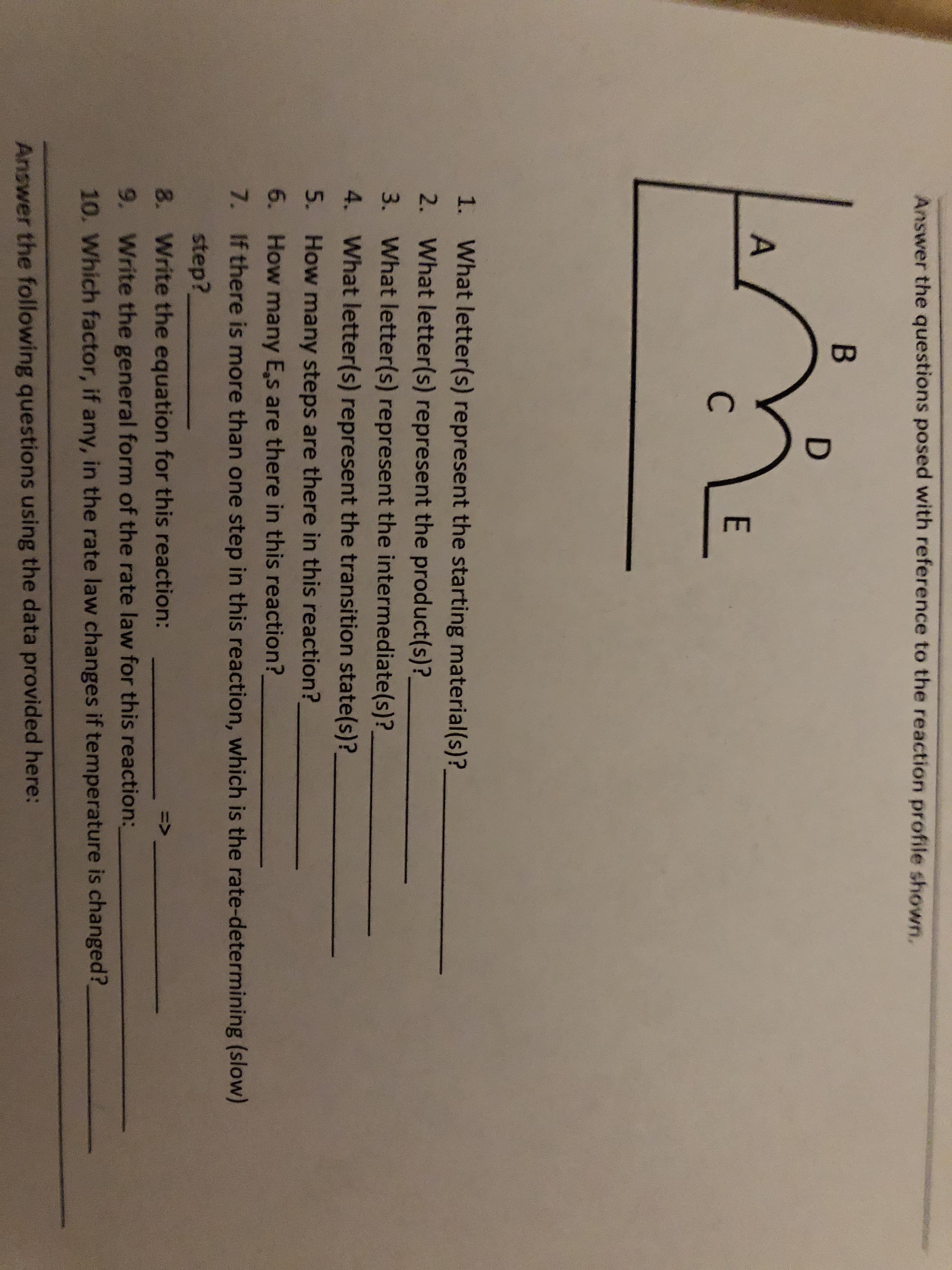
Transcribed Image Text:Answer the questions posed with reference to the reaction profile shown.
В
D
A
E
С
1.
What letter(s) represent the starting material(s)?
2.
What letter(s) represent the product(s)?
3.
What letter(s) represent the intermediate(s)?
4.
What letter(s) represent the transition state(s)?
5.
How many steps are there in this reaction?
6.
How many Es are there in this reaction?
7.
If there is more than one step in this reaction, which is the rate-determining (slow)
step?
8.
Write the equation for this reaction:
9. Write the general form of the rate law for this reaction:
10. Which factor, if any, in the rate law changes if temperature is changed?
Answer the following questions using the data provided here:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning