Approximately 80% of the energy used by the body must be dissipated thermally. The mechanisms available to eliminate this energy are radiation, evaporation of sweat, evaporation from the lungs, conduction, and convection. In this question, we will focus on the evaporation of sweat alone, although all of these mechanisms are needed to survive. The Part A To cool the body of a jogger of mass 85 kg by 1.8°C , how much sweat has to evaporate O 220 g latent heat of vaporization of sweat at body temperature (37° C) is 2.42×106 J/kg and the specific heat of a body is approximately 3500 J/(kg - °C). O 12 g O 22 g O 120 g Submit Request Answer Part B Due to the evaporation of the jogger's sweat, the change in entropy of the universe is: O Zero O Negative O Not able to be determined with the given information O Positive
Approximately 80% of the energy used by the body must be dissipated thermally. The mechanisms available to eliminate this energy are radiation, evaporation of sweat, evaporation from the lungs, conduction, and convection. In this question, we will focus on the evaporation of sweat alone, although all of these mechanisms are needed to survive. The Part A To cool the body of a jogger of mass 85 kg by 1.8°C , how much sweat has to evaporate O 220 g latent heat of vaporization of sweat at body temperature (37° C) is 2.42×106 J/kg and the specific heat of a body is approximately 3500 J/(kg - °C). O 12 g O 22 g O 120 g Submit Request Answer Part B Due to the evaporation of the jogger's sweat, the change in entropy of the universe is: O Zero O Negative O Not able to be determined with the given information O Positive
Chapter1: Temperature And Heat
Section: Chapter Questions
Problem 80P: How many grams of coffee must evaporate from 350 g of coffee in a 100-g glass cup to cool the coffee...
Related questions
Question
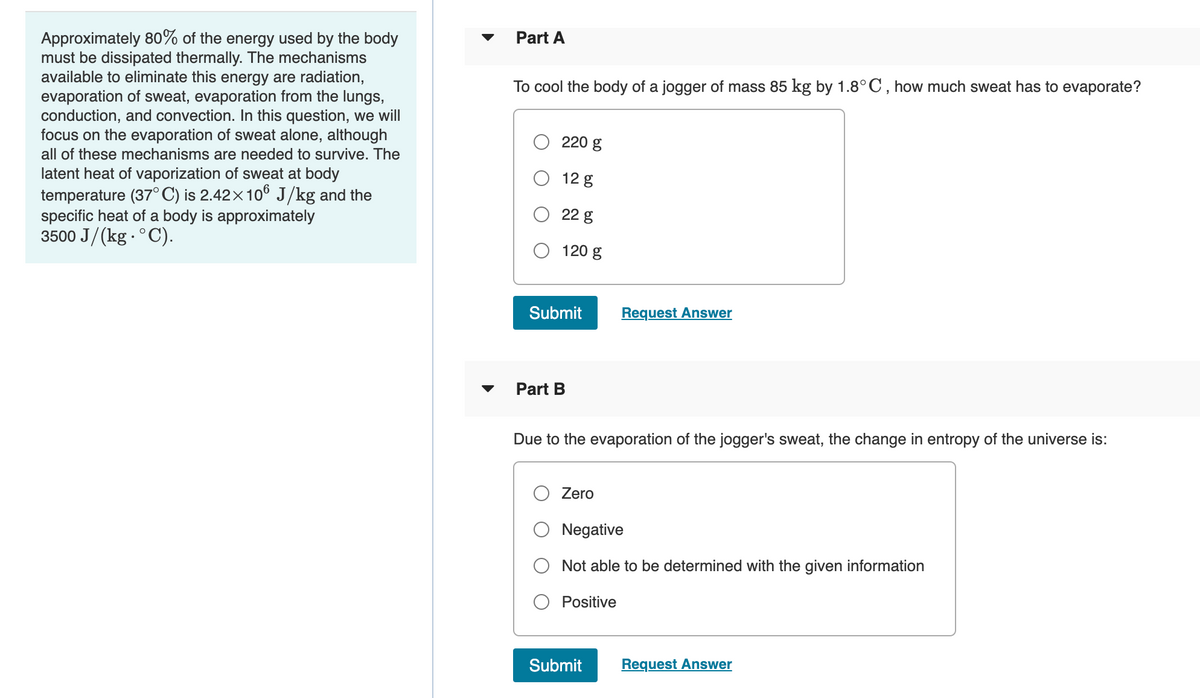
Transcribed Image Text:Approximately 80% of the energy used by the body
must be dissipated thermally. The mechanisms
available to eliminate this energy are radiation,
evaporation of sweat, evaporation from the lungs,
conduction, and convection. In this question, we will
focus on the evaporation of sweat alone, although
all of these mechanisms are needed to survive. The
Part A
To cool the body of a jogger of mass 85 kg by 1.8°C , how much sweat has to evaporate?
220 g
latent heat of vaporization of sweat at body
temperature (37°C) is 2.42×106 J/kg and the
specific heat of a body is approximately
3500 J/(kg - °C).
12 g
22 g
120 g
Submit
Request Answer
Part B
Due to the evaporation of the jogger's sweat, the change in entropy of the universe is:
Zero
Negative
Not able to be determined with the given information
Positive
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning