Apps O THERMOCHEMISTRY O as Calculating specific heat capacity A chemist carefully measures the amount of heat needed to raise the temperature of a 0.67 kg sample of a pure substance from 17.6 °C to 33.6 °C. The experiment shows that 20. kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 significant digits. -1 ·K ? Explanation Check 72022 McGraw Hill LLC. All RIights Reserved. Terms of Use Pioncy Center PIIVNcy Center Accectbl DIMG44040g D ynage 50378449 jPG Image 50371073 JPG MG-A40.ipA MacBook Air DII DO 80 F7 FO F9 esc F4 F5 FI F2 F3 @ $ & 3 4. 5 6. 8
Apps O THERMOCHEMISTRY O as Calculating specific heat capacity A chemist carefully measures the amount of heat needed to raise the temperature of a 0.67 kg sample of a pure substance from 17.6 °C to 33.6 °C. The experiment shows that 20. kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 significant digits. -1 ·K ? Explanation Check 72022 McGraw Hill LLC. All RIights Reserved. Terms of Use Pioncy Center PIIVNcy Center Accectbl DIMG44040g D ynage 50378449 jPG Image 50371073 JPG MG-A40.ipA MacBook Air DII DO 80 F7 FO F9 esc F4 F5 FI F2 F3 @ $ & 3 4. 5 6. 8
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
Transcribed Image Text:O THERMOCHEMISTRY
11
Das
Calculating specific heat capacity
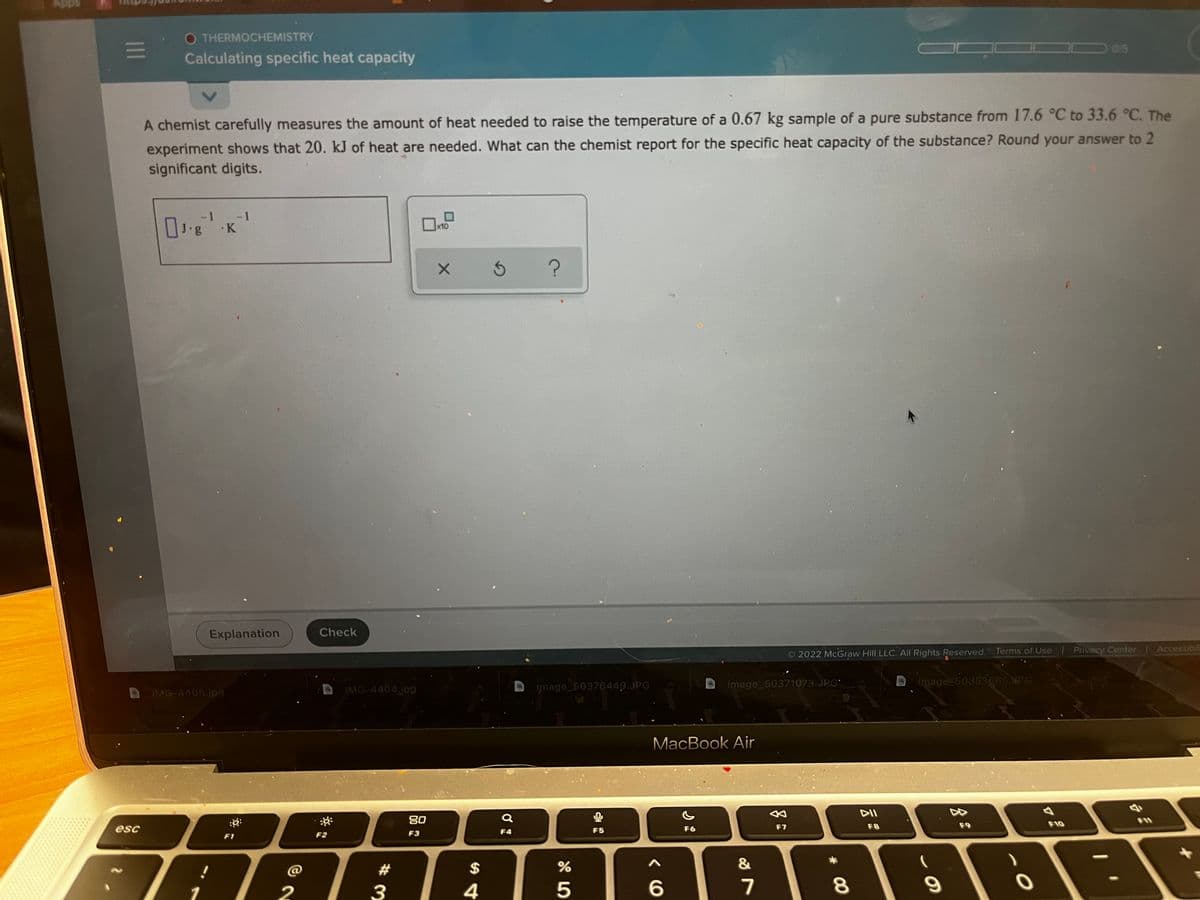
A chemist carefully measures the amount of heat needed to raise the temperature of a 0.67 kg sample of a pure substance from 17.6 °C to 33.6 °C. The
experiment shows that 20. kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2
significant digits.
-1
J.g
-1
x10
Explanation
Check
2022 McGraw Hill LLC. All Rights Reserved.
Terms of Use Privacy Center Accessibile
image 50371073.JPG:
Image 50353665.3PG
IMG-4404jpg
ijmage_50376449.JPG
IMG-4405.jpg
MacBook Air
DII
80
F11
F7
F8
F9
F10
esc
F5
F6
F2
F3
F4
F1
@
23
%24
1
3
4
7
* 00
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning