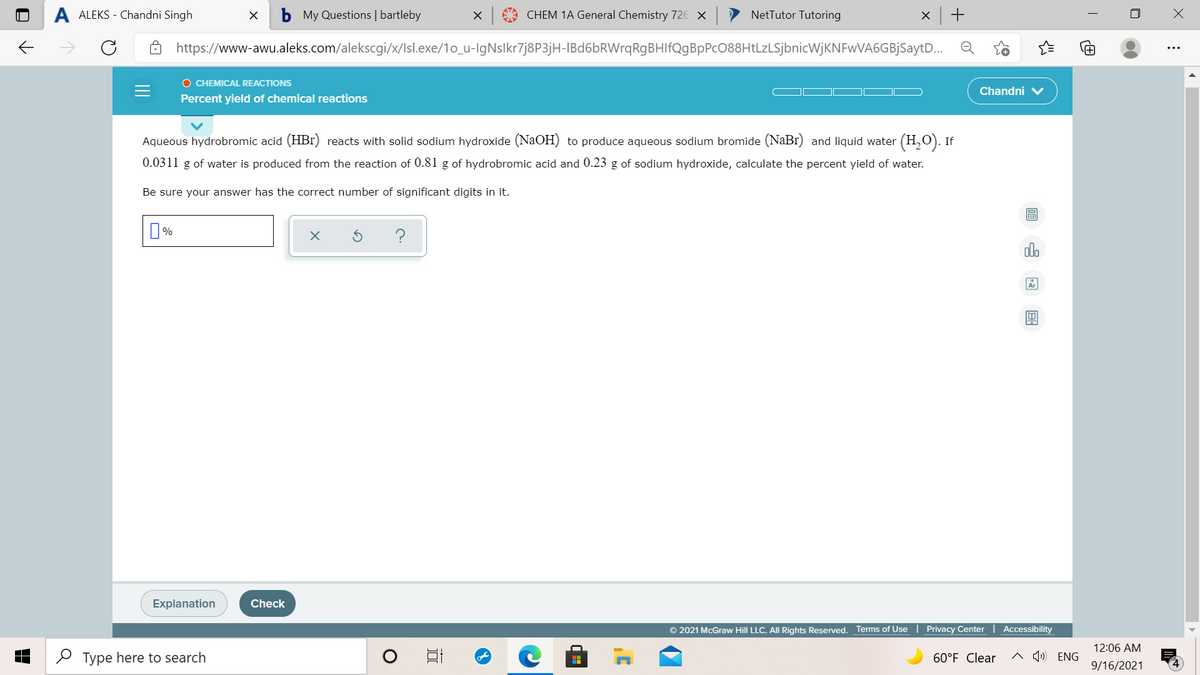
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,0). If 0.0311 g of water is produced from the reaction of 0.81 g of hydrobromic acid and 0.23 g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. do
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,0). If 0.0311 g of water is produced from the reaction of 0.81 g of hydrobromic acid and 0.23 g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. do
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter32: Radiochemical Methods
Section: Chapter Questions
Problem 32.7QAP
Related questions
Question
Transcribed Image Text:ALEKS - Chandni Singh
My Questions | bartleby
X 9 CHEM 1A General Chemistry 726 X
NetTutor Tutoring
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBd6bRWrqRgBHIfQgBpPcO88HtLzLSjbnicWjKNFwVA6GBjSaytD..
O CHEMICAL REACTIONS
Chandni
Percent yield of chemical reactions
Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). If
0.0311 g of water is produced from the reaction of 0.81 g of hydrobromic acid and 0.23 g of sodium hydroxide, calculate the percent yield of water.
Be sure your answer has the correct number of significant digits in it.
圖
%
olo
Explanation
Check
© 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
12:06 AM
2 Type here to search
60°F Clear
^ Q») ENG
9/16/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning