Aqueous osmium (II) chloride reacts with aqueous cesium carbonate to produce cesium chloride and osmium (II) carbonate. (Osmium has the symbol Os) Solubility Rules Solubility Exceptions Solubility Exceptions insoluble Li", Na", K* , Rb*, Cs*, NH, lon lon nitrates soluble none carbonates sulfates soluble Ca* ,Ba" ,Sr* , Ag" , Pb* insoluble Li, Na', K* , Rb*, Cs', NH. phosphates chlorides soluble Ba?", Sr?* insoluble Li , Na', K' , Rb', Cs', NH, *, Ca insoluble Li , Na', K' , Rb', Cs', NH, ', Mg' Ag, Pb hydroxides alkali ions soluble none sulfides Ca Ва acetates soluble none iodides soluble Ag, Pb* 1) Write the balance equation including phases (s, I, aq etc.) on the paper with your work that you will submit after the exam. 2) Enter the coefficient in front of the aluminum chloride when the equation is balanced in moodle. The equation you submit must support the number you entered in moodle. Select one: O a. 6 b. 2 с. 5 d. 3 О е.1 O f. 4
Aqueous osmium (II) chloride reacts with aqueous cesium carbonate to produce cesium chloride and osmium (II) carbonate. (Osmium has the symbol Os) Solubility Rules Solubility Exceptions Solubility Exceptions insoluble Li", Na", K* , Rb*, Cs*, NH, lon lon nitrates soluble none carbonates sulfates soluble Ca* ,Ba" ,Sr* , Ag" , Pb* insoluble Li, Na', K* , Rb*, Cs', NH. phosphates chlorides soluble Ba?", Sr?* insoluble Li , Na', K' , Rb', Cs', NH, *, Ca insoluble Li , Na', K' , Rb', Cs', NH, ', Mg' Ag, Pb hydroxides alkali ions soluble none sulfides Ca Ва acetates soluble none iodides soluble Ag, Pb* 1) Write the balance equation including phases (s, I, aq etc.) on the paper with your work that you will submit after the exam. 2) Enter the coefficient in front of the aluminum chloride when the equation is balanced in moodle. The equation you submit must support the number you entered in moodle. Select one: O a. 6 b. 2 с. 5 d. 3 О е.1 O f. 4
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter4: Stoichiometry Of Chemical Reactions
Section: Chapter Questions
Problem 86E: Sodium bicarbonate (baking soda), NaHCO3, can be purified by dissolving it in hot water (60 C),...
Related questions
Question
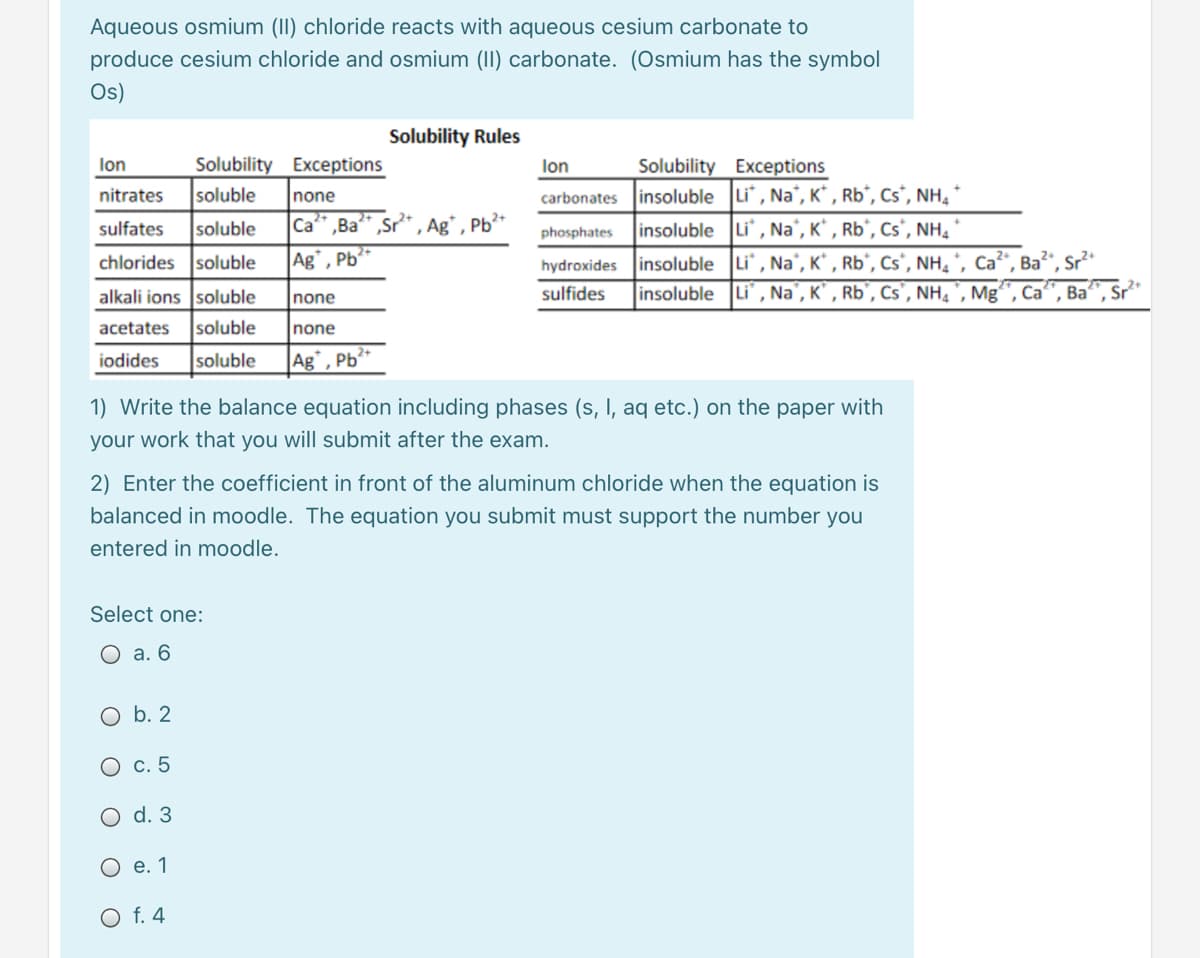
Transcribed Image Text:Aqueous osmium (II) chloride reacts with aqueous cesium carbonate to
produce cesium chloride and osmium (II) carbonate. (Osmium has the symbol
Os)
Solubility Rules
Solubility Exceptions
Solubility Exceptions
insoluble Li", Na", K* , Rb*, Cs*, NH,
lon
lon
nitrates
soluble
none
carbonates
sulfates
soluble
Ca* ,Ba* ,Sr* , Ag* , Pb*
insoluble Li, Na', K* , Rb°, Cs*, NH4
phosphates
chlorides soluble
Ba?", Sr*
insoluble Li , Na', K* , Rb°, Cs', NH, *, Ca
insoluble Li , Na', K' , Rb°, Cs', NH, ', Mg'
Ag°, Pb*
hydroxides
alkali ions soluble
Inone
sulfides
Ca
Ва
acetates
soluble
Inone
iodides
soluble
Ag", Pb²*
1) Write the balance equation including phases (s, I, aq etc.) on the paper with
your work that you will submit after the exam.
2) Enter the coefficient in front of the aluminum chloride when the equation is
balanced in moodle. The equation you submit must support the number you
entered in moodle.
Select one:
О а. 6
b. 2
с. 5
d. 3
О е.1
O f. 4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning