Aqueous sodium oxalate can react with aqueous calcium citrate [Ca3(CoHsO»)l to produce solid calcium oxalate and aqueous sodium citrate (NagCHsO). For the reaction described above, which of the following is the correct balanced chemical equation including phases? O 3Na,C,O4 + Cag(C,H;O)2 --> 2Na,CHsO, + 3CAC,04 O 3Na2C,O4 (aq) + Cag(CH5O)2 (aq) --> 2N33C,HgOy (aq) + 3CaC,O, (s) O 3NACO4 (aq) + Cag(CHSO)2 (aq) --> 2NaC,HsO, (aq) + 3CAC,O, (s) O 6NAC,O, laq) + 2Cag(CH,O)2 (aq) --> 4NACH3O7 (aq) + 6CaC,0, (5) O Nac,O. (aq) + CaCHsO, (aq) --> NaCgHsO, (aq) + CaC,O (s) The major component of most kidney stones is calcium oxalate. Aqueous sodium oxalate can react with aqueous calcium citrate (Cas(CHsO2 to produce solid calcium oxalate and aqueous sodium citrate (NazCHsO-). For the reaction described above, what is the precipitate? O NaC2O4 (s) O NaC,HsO, (s) O Cac204 (s) O Cag(CH5O7)2 (s) O NazCHsO,(s) O NazC204 (s)
Aqueous sodium oxalate can react with aqueous calcium citrate [Ca3(CoHsO»)l to produce solid calcium oxalate and aqueous sodium citrate (NagCHsO). For the reaction described above, which of the following is the correct balanced chemical equation including phases? O 3Na,C,O4 + Cag(C,H;O)2 --> 2Na,CHsO, + 3CAC,04 O 3Na2C,O4 (aq) + Cag(CH5O)2 (aq) --> 2N33C,HgOy (aq) + 3CaC,O, (s) O 3NACO4 (aq) + Cag(CHSO)2 (aq) --> 2NaC,HsO, (aq) + 3CAC,O, (s) O 6NAC,O, laq) + 2Cag(CH,O)2 (aq) --> 4NACH3O7 (aq) + 6CaC,0, (5) O Nac,O. (aq) + CaCHsO, (aq) --> NaCgHsO, (aq) + CaC,O (s) The major component of most kidney stones is calcium oxalate. Aqueous sodium oxalate can react with aqueous calcium citrate (Cas(CHsO2 to produce solid calcium oxalate and aqueous sodium citrate (NazCHsO-). For the reaction described above, what is the precipitate? O NaC2O4 (s) O NaC,HsO, (s) O Cac204 (s) O Cag(CH5O7)2 (s) O NazCHsO,(s) O NazC204 (s)
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.21EP: Classify each of the following reactions as (1) a redox reaction (2) a nonredox reaction or (3) cant...
Related questions
Question
6.
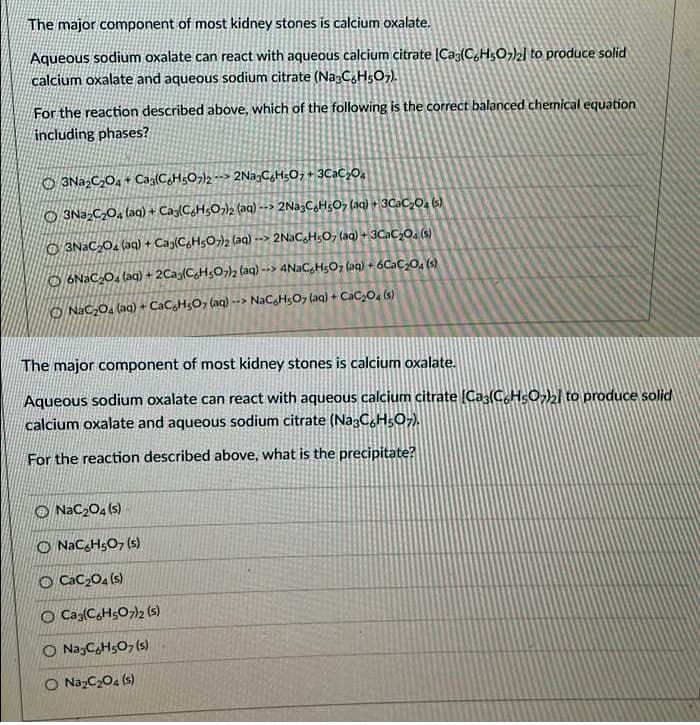
Transcribed Image Text:The major component of most kidney stones is calcium oxalate.
Aqueous sodium oxalate can react with aqueous calcium citrate (Caz(C,HsO,)zl to produce solid
calcium oxalate and aqueous sodium citrate (Na:C,H5O7).
For the reaction described above, which of the following is the correct balanced chemical equation
including phases?
O 3Na,C,O, + Cag(C,H;O¬)2 --> 2Na,CH;O; + 3C2C,0.
O 3NazC204 (aa) + Cag(CH5O)2 (aq) --> 2Na3CaHs0, (aq) + 3CAC,0, (s)
O 3NACO2 (aq) + Cag(C,H5O2 (aq) --> 2N3C,HsO, (aq) + 3CaC,O. (s)
O 6NAC,O, (ag) + 2Cag(C,H,O)2 (aq) --> 4NaCgHgOz (aq) + 6Cac,0, ()
O NaC,O, (aq) + CaCgHsO, (aq) --> NaCgHsO7 (aq) + CaC2O4 (s)
The major component of most kidney stones is calcium oxalate.
Aqueous sodium oxalate can react with aqueous calcium citrate (Cas(C,HsO2l to produce solid
calcium oxalate and aqueous sodium citrate (Na3C.HsO-).
For the reaction described above, what is the precipitate?
O NaC204 (s)
O NaC,HsO, (s)
O CaC204 (s)
O Ca{CH5O7)2 (s)
O NazCHs0, (s)
O NazC204 (s)
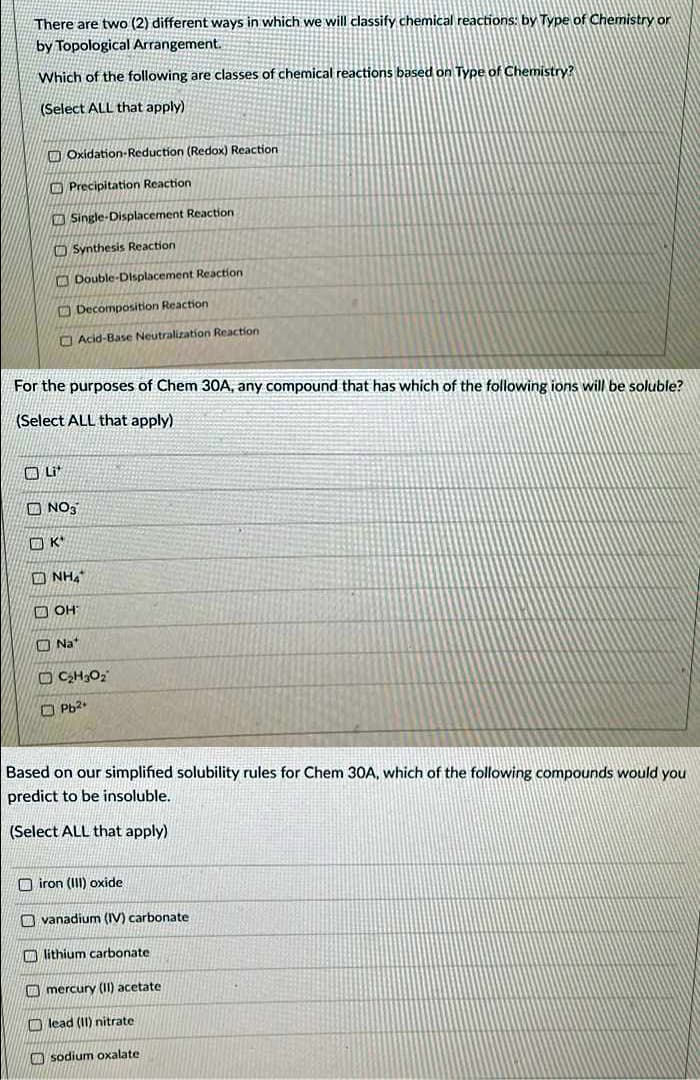
Transcribed Image Text:There are two (2) different ways in which we will classify chemical reactions: by Type of Chemistry or
by Topological Arrangement.
Which of the following are classes of chemical reactions based on Type of Chemistry?
(Select ALL that apply)
O Oxidation-Reduction (Redox) Reaction
O Precipitation Reaction
Single-Displacement Reaction
O Synthesis Reaction
O Double-Dlsplacement Reaction
O Decomposition Reaction
O Acid-Base Neutralization Reaction
For the purposes of Chem 30A, any compound that has which of the following ions will be soluble?
(Select ALL that apply)
O NO3
O K*
O NH,
O OH
O Na*
O Pb2
Based on our simplified solubility rules for Chem 30A, which of the following compounds would you
predict to be insoluble.
(Select ALL that apply)
O iron (III) oxide
O vanadium (IV) carbonate
O lithium carbonate
O mercury (11) acetate
O lead (II) nitrate
O sodium oxalate
O 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning