AR a - 4CDU-AD, 4CBa How many grams of AD, IsMW-285.72 g/mol would be made from the reaction of 2.18E24 molecules of CD la q) (MW-295.97 g/mol), assuming excess AB la q) 1279.92 g/mol0? (given Avogadro's number-6.022E23) Your Answer Answer units Solution Question 29 It is observed that 33.90 g of NaCI (MW 58.44 g/mol) dissolves in 100.0 mL of water at 50 degrees celsius. What is the molarity of NaCl in the solution? Assume that the volume of the solution does not change as the NaCl is added.
AR a - 4CDU-AD, 4CBa How many grams of AD, IsMW-285.72 g/mol would be made from the reaction of 2.18E24 molecules of CD la q) (MW-295.97 g/mol), assuming excess AB la q) 1279.92 g/mol0? (given Avogadro's number-6.022E23) Your Answer Answer units Solution Question 29 It is observed that 33.90 g of NaCI (MW 58.44 g/mol) dissolves in 100.0 mL of water at 50 degrees celsius. What is the molarity of NaCl in the solution? Assume that the volume of the solution does not change as the NaCl is added.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 64A
Related questions
Question
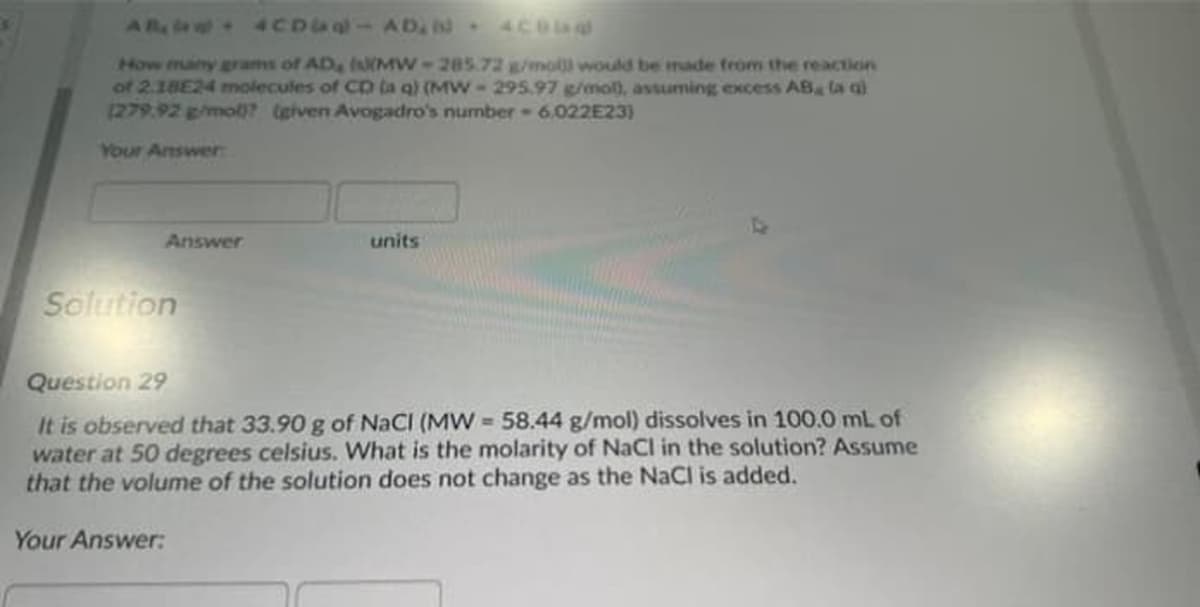
Transcribed Image Text:ARa - 4CDU-AD, 6 4Coa
How many grams of AD, IsMW-285.72 g/mol would be made from the reaction
of 2.18E24 molecules of CD la q) (MW-295.97 g/mol), assuming excess AB la g)
1279.92 g/mol)? (given Avogadro's number 6.022E23)
Your Answer
Answer
units
Solution
Question 29
It is observed that 33.90 g of NaCI (MW 58.44 g/mol) dissolves in 100.0 mL of
water at 50 degrees celsius. What is the molarity of NaCl in the solution? Assume
that the volume of the solution does not change as the NaCl is added.
Your Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning