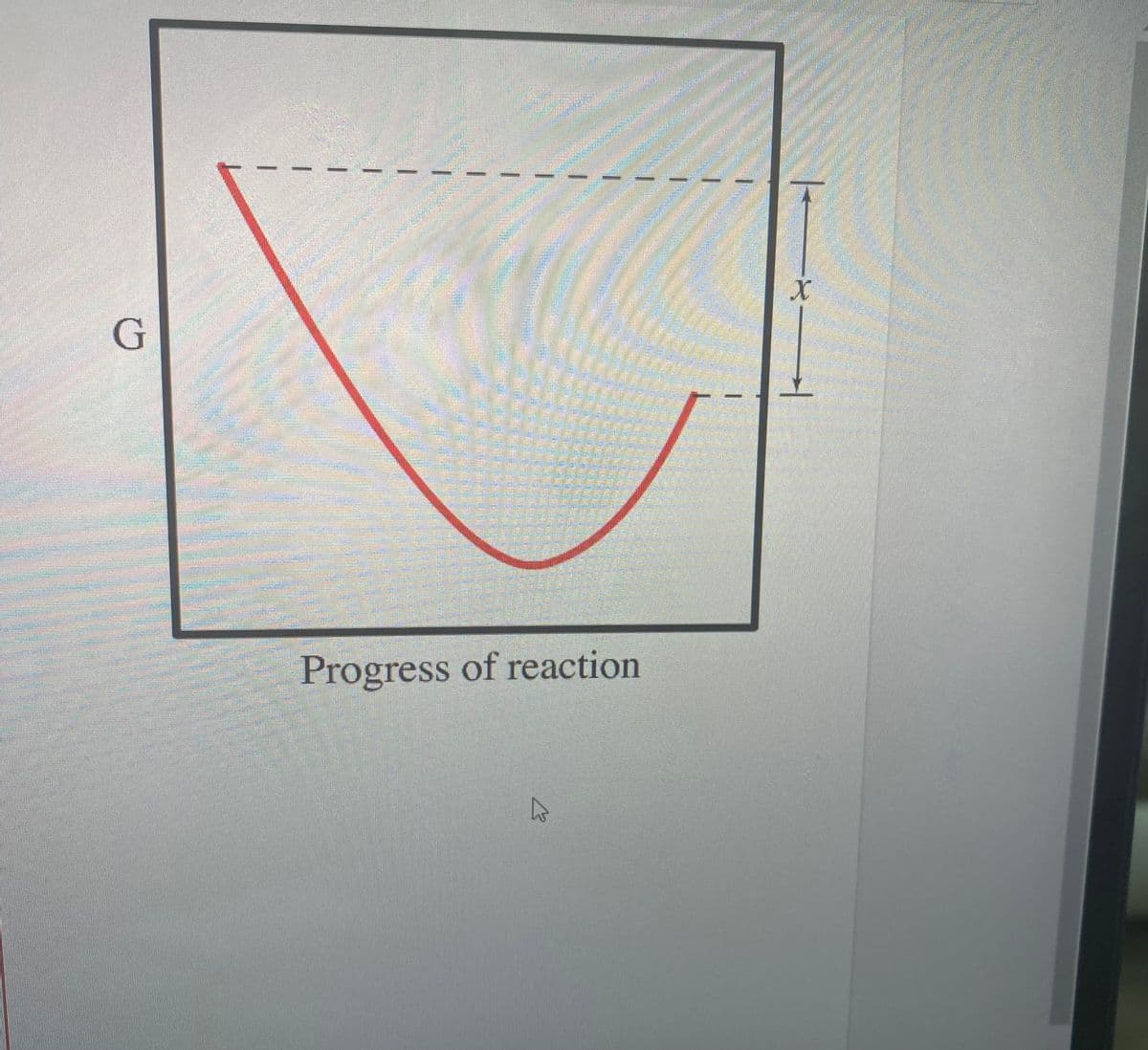
Select the true statements. The x on the graph corresponds to AG of this reaction. The minimum on the graph corresponds to the equilibrium position of the reaction. The entropy change for the reaction is positive. The difference between the top left of the curve and the bottom of the curve corresponds to AG of this reaction. At equilibrium, all of A and B have reacted to form pure AB.
Select the true statements. The x on the graph corresponds to AG of this reaction. The minimum on the graph corresponds to the equilibrium position of the reaction. The entropy change for the reaction is positive. The difference between the top left of the curve and the bottom of the curve corresponds to AG of this reaction. At equilibrium, all of A and B have reacted to form pure AB.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter13: Substitution
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
The diagram shows the free energy change of the reaction
A(g)+B(g)↽−−⇀AB(g)
The reaction progress starts on the left with pure reactants, A and B, each at 1 atm, and moves to pure product, AB, which is at 1 atm on the right.
Select the true statements

Transcribed Image Text:Select the true statements.
The x on the graph corresponds to
AG of this reaction.
The minimum on the graph corresponds to the
equilibrium position of the reaction.
The entropy change for the reaction is positive.
The difference between the top left of the curve and
the bottom of the curve corresponds to
AG of this reaction.
At equilibrium, all of A and B have reacted to form
pure AB.
Transcribed Image Text:G
Pag
CAMERON
Progress of reaction
4
75
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
I tried both suggestions and they were both incorrect. Not sure what else to try
Solution
Follow-up Question
I submitted these responses and they were incorrect. Are there any other suggestions?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

