Arrange the following in order of increasing boiling point: CH3CH3, CH3CH2CH3, CH3CH2CH2C1, CH3CH2CH2OH. Rank from smallest to largest. To rank items as equivalent, overlap them. Reset Help CH3CH2CH2CI CH3CH3 CH3CH2 CH2OH CH3CH2CH3 lowest boiling point highest boiling point
Arrange the following in order of increasing boiling point: CH3CH3, CH3CH2CH3, CH3CH2CH2C1, CH3CH2CH2OH. Rank from smallest to largest. To rank items as equivalent, overlap them. Reset Help CH3CH2CH2CI CH3CH3 CH3CH2 CH2OH CH3CH2CH3 lowest boiling point highest boiling point
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter15: Carboxylic Acids And Esters
Section: Chapter Questions
Problem 15.74E
Related questions
Question
I need help please
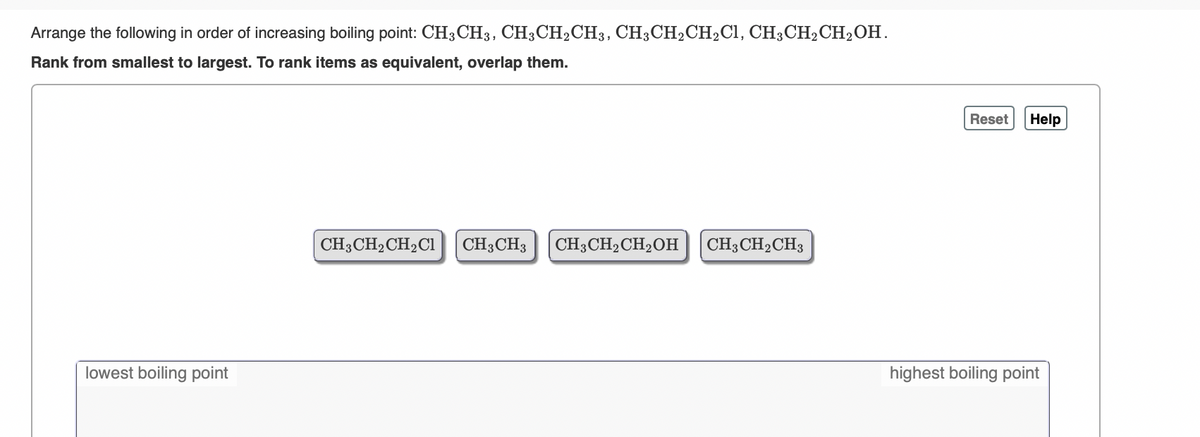
Transcribed Image Text:Arrange the following in order of increasing boiling point: CH3CH3, CH3CH2CH3, CH;CH2CH2C1, CH3CH2CH2OH.
Rank from smallest to largest. To rank items as equivalent, overlap them.
Reset
Help
CH3CH2CH2CI
CH3CH3
CH3CH2CH2OH| CH3CH2CH3
lowest boiling point
highest boiling point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning