Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 141QRT
Related questions
Question
Answer only the table with no answers
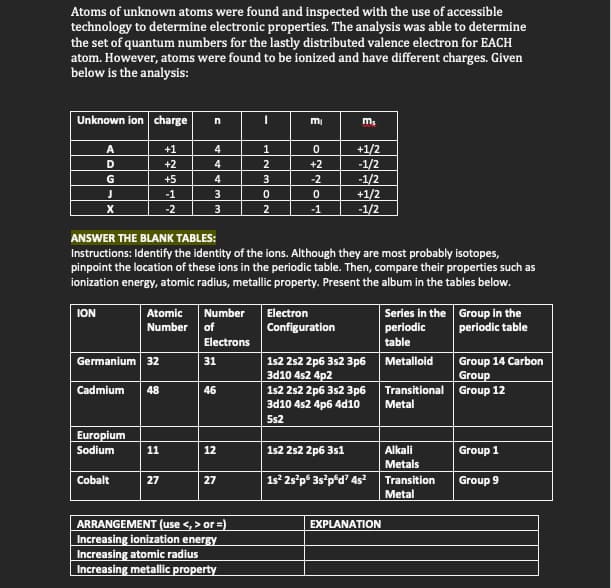
Transcribed Image Text:Atoms of unknown atoms were found and inspected with the use of accessible
technology to determine electronic properties. The analysis was able to determine
the set of quantum numbers for the lastly distributed valence electron for EACH
atom. However, atoms were found to be ionized and have different charges. Given
below is the analysis:
Unknown ion charge
mi
+1/2
-1/2
+1
1
D
+2
4
+2
-1/2
+1/2
G
+5
-2
-1
3
-2
3
2
-1
-1/2
ANSWER THE BLANK TABLES:
Instructions: Identify the identity of the ions. Although they are most probably isotopes,
pinpoint the location of these ions in the periodic table. Then, compare their properties such as
ionization energy, atomic radius, metallic property. Present the album in the tables below.
Number
Electron
Series in the Group in the
periodic
ION
Atomic
Number of
Configuration
periodic table
Electrons
table
Germanium 32
1s2 2s2 2p6 3s2 3p6
3d10 4s2 4p2
1s2 2s2 2p6 3s2 3p6
3d10 4s2 4p6 4d10
Group 14 Carbon
Group
Transitional Group 12
31
Metalloid
Cadmium
48
46
Metal
5s2
Europium
Sodium
11
12
1s2 2s2 2p6 3s1
Alkali
Group 1
Metals
Cobalt
1s² 25°p° 3s°p°d? 4s?
27
27
Transition
Group 9
Metal
ARRANGEMENT (use <, > or =)
Increasing ionization energy
Increasing atomic radius
Increasing metallic property
EXPLANATION
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning