Assess the validity of each statement and choose X if the statement is CORRECT, Choose Y if otherwise. 1. Water, ammonia and halide ions are inorganic ligands. 2. Ligands are acceptors. 3. Complexation reactions involve a metal-ion reacting with a ligand
Assess the validity of each statement and choose X if the statement is CORRECT, Choose Y if otherwise. 1. Water, ammonia and halide ions are inorganic ligands. 2. Ligands are acceptors. 3. Complexation reactions involve a metal-ion reacting with a ligand
Chapter9: Complexometric And Precipitation Titrations
Section: Chapter Questions
Problem 7P
Related questions
Question
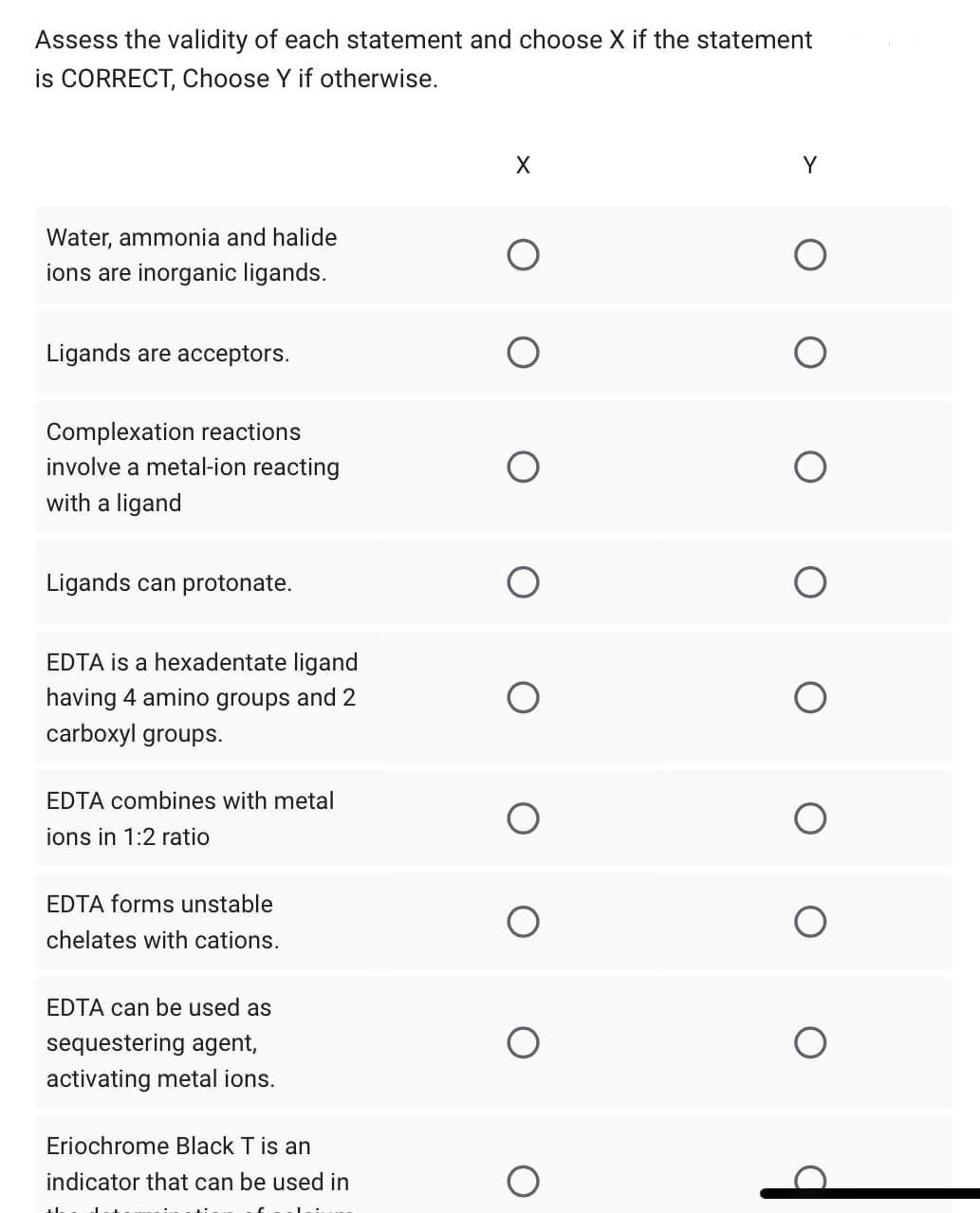
Assess the validity of each statement and choose X if the statement is CORRECT, Choose Y if otherwise.
1. Water, ammonia and halide ions are inorganic ligands.
2. Ligands are acceptors.
3. Complexation reactions involve a metal-ion reacting with a ligand
4. Ligands can protonate.
5. EDTA is a hexadentate ligand having 4 amino groups and 2 carboxyl groups.
6. EDTA combines with metal ions in 1:2 ratio
7. EDTA forms unstable chelates with cations.
8. EDTA can be used as sequestering agent, activating metal ions.
9. Eriochrome Black T is an indicator that can be used in the determination of calcium.
Transcribed Image Text:Assess the validity of each statement and choose X if the statement
is CORRECT, Choose Y if otherwise.
Water, ammonia and halide
ions are inorganic ligands.
Ligands are acceptors.
Complexation reactions
involve a metal-ion reacting
with a ligand
Ligands can protonate.
EDTA is a hexadentate ligand
having 4 amino groups and 2
carboxyl groups.
EDTA combines with metal
ions in 1:2 ratio
EDTA forms unstable
chelates with cations.
EDTA can be used as
sequestering agent,
activating metal ions.
Eriochrome Black T is an
indicator that can be used in
X
ο ο ο
O
Y
O
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning