8. Which of the following reactions is a redox reaction? A) 2HCl + Ca(OH)2 → CaCl2 + 2H₂O B)CaCO3 → CaO + CO2 C) HCl + AgNO3 → AgCl + HNO3 D) 2PbO2 → 2PbO + 0₂ E) NaHCO3 + H₂SO4 → Na2SO4 + CO₂ + H₂O
8. Which of the following reactions is a redox reaction? A) 2HCl + Ca(OH)2 → CaCl2 + 2H₂O B)CaCO3 → CaO + CO2 C) HCl + AgNO3 → AgCl + HNO3 D) 2PbO2 → 2PbO + 0₂ E) NaHCO3 + H₂SO4 → Na2SO4 + CO₂ + H₂O
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
100%
Hey i need some help :)) (no diagram or explanation needed)
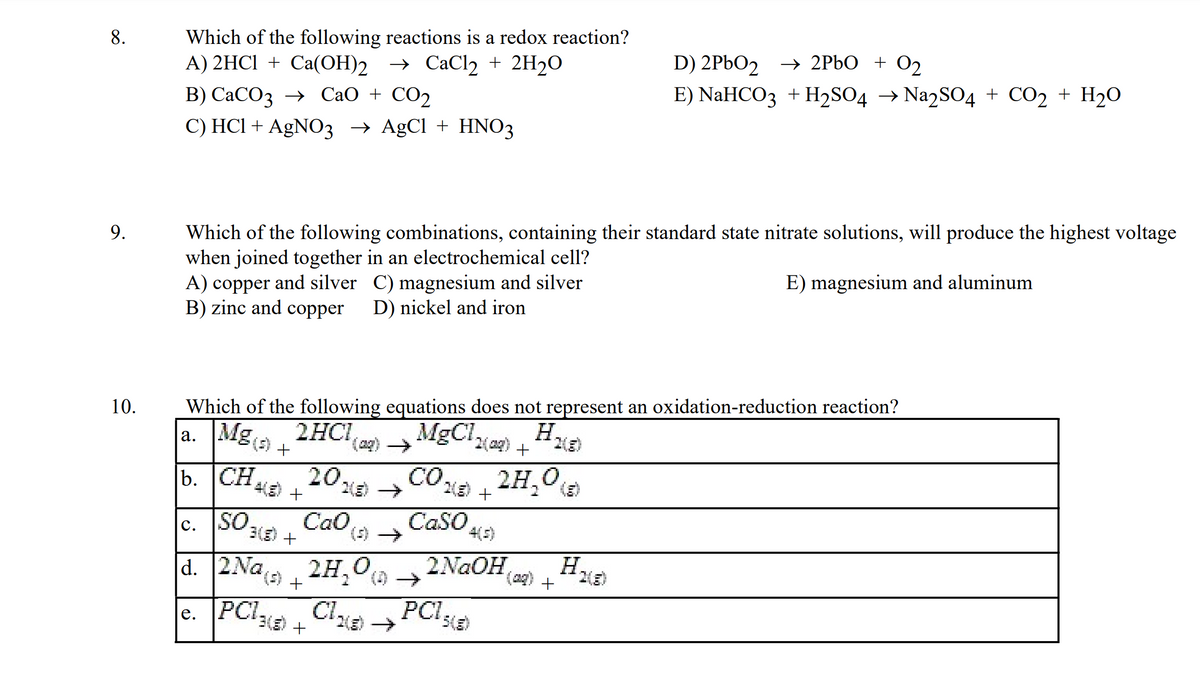
Transcribed Image Text:8.
9.
10.
Which of the following reactions is a redox reaction?
A) 2HCl + Ca(OH)2
Ca(OH)2 → CaCl₂ + 2H₂O
B) CaCO3 → CaO + CO2
C) HCl + AgNO3 → AgCl + HNO3
Which of the following combinations, containing their standard state nitrate solutions, will produce the highest voltage
when joined together in an electrochemical cell?
E) magnesium and aluminum
A) copper and silver C) magnesium and silver
B) zinc and copper
D) nickel and iron
Which of the following equations does not represent an oxidation-reduction reaction?
a. Mg(s) +
2HC1(₂)→
MgCln(aq) + H.
b.
2H₂0
2028) →
Cao
CH 4(E) +
SO 3(E) +
C.
d. 2Na(s) +
e.
CO₂5) +
CaSO
27,0 € +
PC1)Cl₂(8)→
+
4(5)
2NaOH
PC15(8)
(20)
D) 2PbO2 → 2PbO + O₂
E) NaHCO3 + H₂SO4 → Na2SO4 + CO2 + H₂O
+
H.
H₂(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning