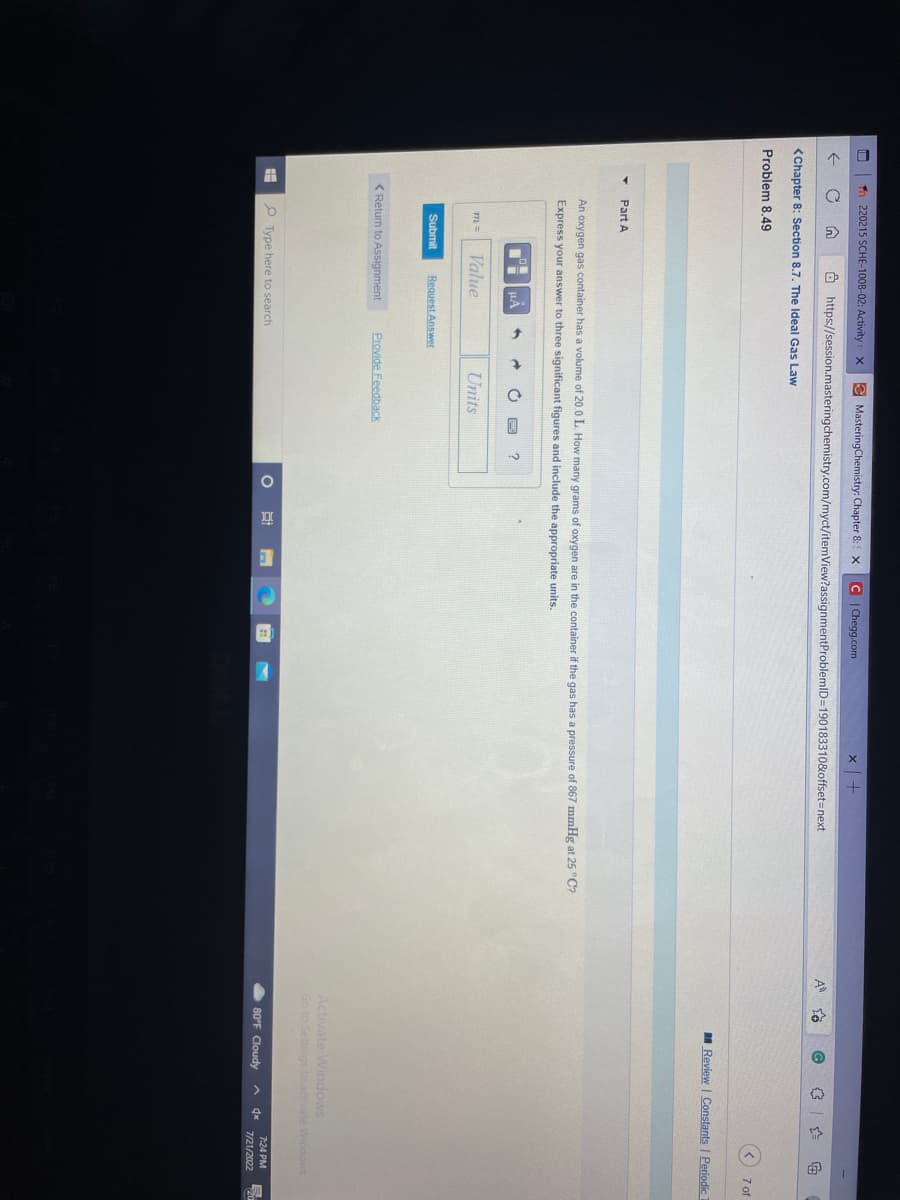
Y Part A An oxygen gas container has a volume of 20.0 L. How many grams of oxygen are in the container if the gas has a pressure of 867 mmHg at 25 °C? Express your answer to three significant figures and include the appropriate units. m = Submit OM ILA Value Request Answer ➜ Units ?
Y Part A An oxygen gas container has a volume of 20.0 L. How many grams of oxygen are in the container if the gas has a pressure of 867 mmHg at 25 °C? Express your answer to three significant figures and include the appropriate units. m = Submit OM ILA Value Request Answer ➜ Units ?
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.14E
Related questions
Question
Transcribed Image Text:←
Th 220215 SCHE-1008-02: Activity
O
H
<Chapter 8: Section 8.7. The Ideal Gas Law
Problem 8.49
Part A
MasteringChemistry: Chapter 8: X C | Chegg.com
https://session.mastering chemistry.com/myct/itemView?assignment
m =
An oxygen gas container has a volume of 20.0 L. How many grams of oxygen are in the container if the gas has a pressure of 867 mmHg at 25 °C?
Express your answer to three significant figures and include the appropriate units.
μÅ
Value
Submit Request Answer
< Return to Assignment
1
Type here to search
Units
Provide Feedback
?
O
X +
B
ProblemID=190183310&offset=next
A
3
G
3
<7 of
Review | Constants | Periodic
80°F Cloudy
Activate Windows
Go to Settings to activate Windows.
7:24 PM
7/21/2022 520
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning