Assume that an enzyme-catalyzed reaction follows the scheme shown: E + S ES E + P k₁ = 1 x 10%/M-s k-₁ =2.5 x 10°/s k₂= 3.4 x 107/s What is the dissociation constant for the enzyme-substrate, Ks? What is the Michaelis constant, Km, for this enzyme? What is the turnover number, Kcat, for this enzyme? What is the catalytic efficiency for the enzyme? If the initial Et concentration is 0.25mM, what is Vmax?
Assume that an enzyme-catalyzed reaction follows the scheme shown: E + S ES E + P k₁ = 1 x 10%/M-s k-₁ =2.5 x 10°/s k₂= 3.4 x 107/s What is the dissociation constant for the enzyme-substrate, Ks? What is the Michaelis constant, Km, for this enzyme? What is the turnover number, Kcat, for this enzyme? What is the catalytic efficiency for the enzyme? If the initial Et concentration is 0.25mM, what is Vmax?
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter23: Fatty Acid Catabolism
Section: Chapter Questions
Problem 21P: Using the ActiveModel for enoyl-CoA dehydratase, give an example of a case in which conserved...
Related questions
Question
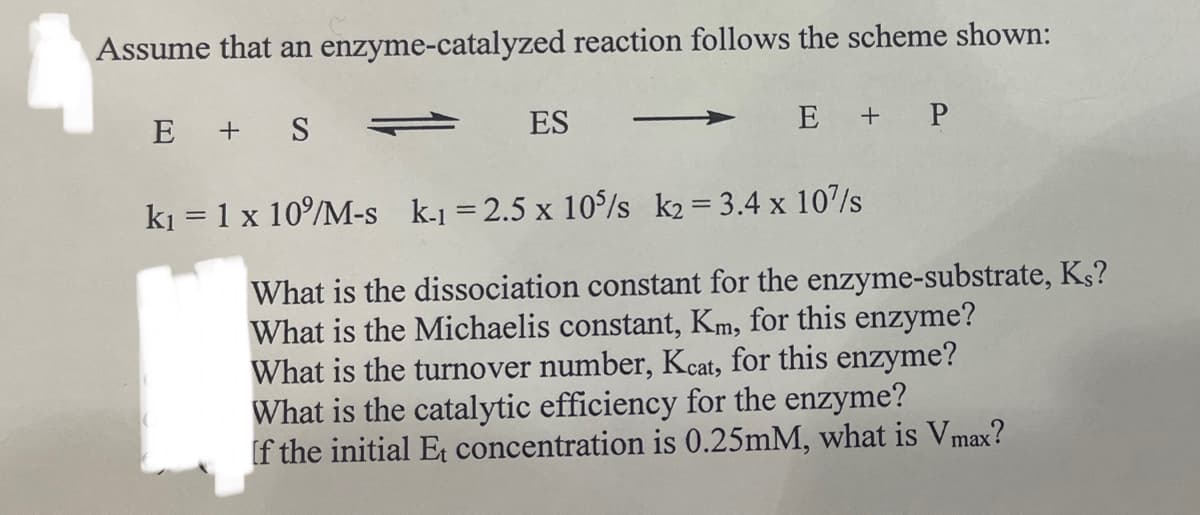
Transcribed Image Text:Assume that an enzyme-catalyzed reaction follows the scheme shown:
E + S
ES
E + P
k₁ = 1 x 10%/M-s k-1 = 2.5 x 10%/s k2= 3.4 x 107s
What is the dissociation constant for the enzyme-substrate, Ks?
What is the Michaelis constant, Km, for this enzyme?
What is the turnover number, Kcat, for this enzyme?
What is the catalytic efficiency for the enzyme?
If the initial Et concentration is 0.25mM, what is Vmax?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: MICHAELIS-MENTEN EQUATION
VIEWStep 2: Calculation of the dissociation constant for the enzyme substrate, Ks
VIEWStep 3: Calculation of Km
VIEWStep 4: Calculation of turnover number Kcat.
VIEWStep 5: Calculation of catalytic efficiency for the enzyme.
VIEWStep 6: Calculation of Vmax
VIEWSolution
VIEWStep by step
Solved in 7 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning