Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
The electrical conductivity of 3 different solutions in 3 different beakers were measured using a lightbulb. In terms of intensity (brightness) of the light bulb:
beaker 3 > beaker 1 > beaker 2.
Assuming equal solution concentrations, what could be the possible identities of the solutions in each of the beakers?
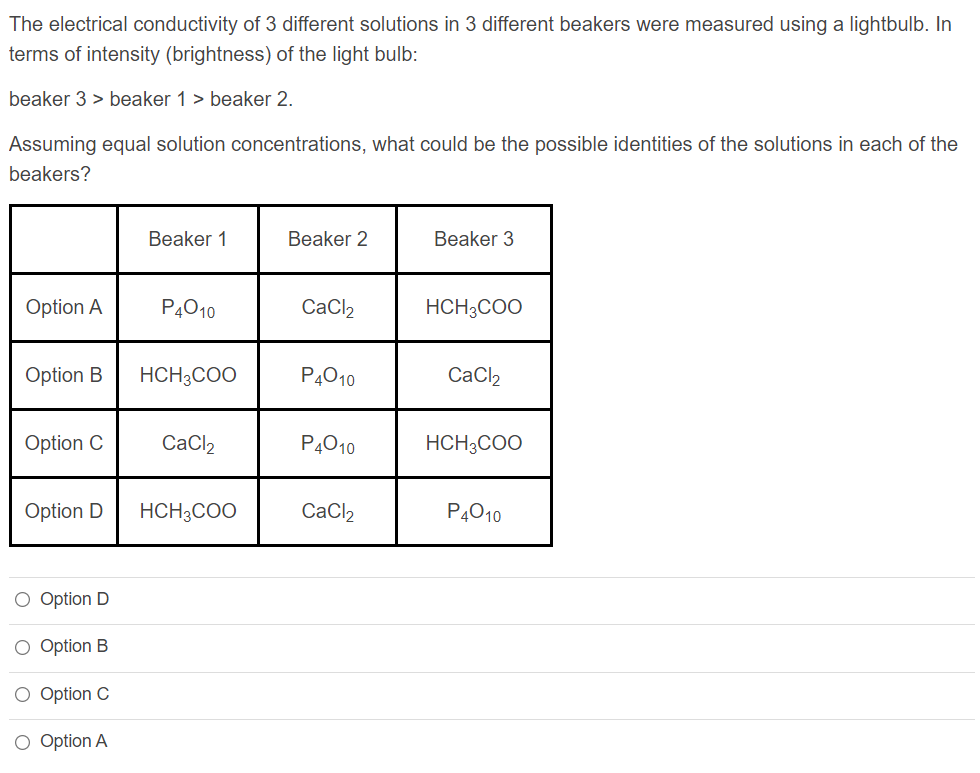
Transcribed Image Text:The electrical conductivity of 3 different solutions in 3 different beakers were measured using a lightbulb. In
terms of intensity (brightness) of the light bulb:
beaker 3 > beaker 1 > beaker 2.
Assuming equal solution concentrations, what could be the possible identities of the solutions in each of the
beakers?
Beaker 1
Beaker 2
Beaker 3
Option A
P4010
CaCl,
HCH3COO
Option B
HCH3COO
P4010
CaCl2
Option C
CaCl2
P4010
HCH3COO
Option D
HCH3COO
CaCl2
P4010
O Option D
O Option B
O Option C
O Option A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co