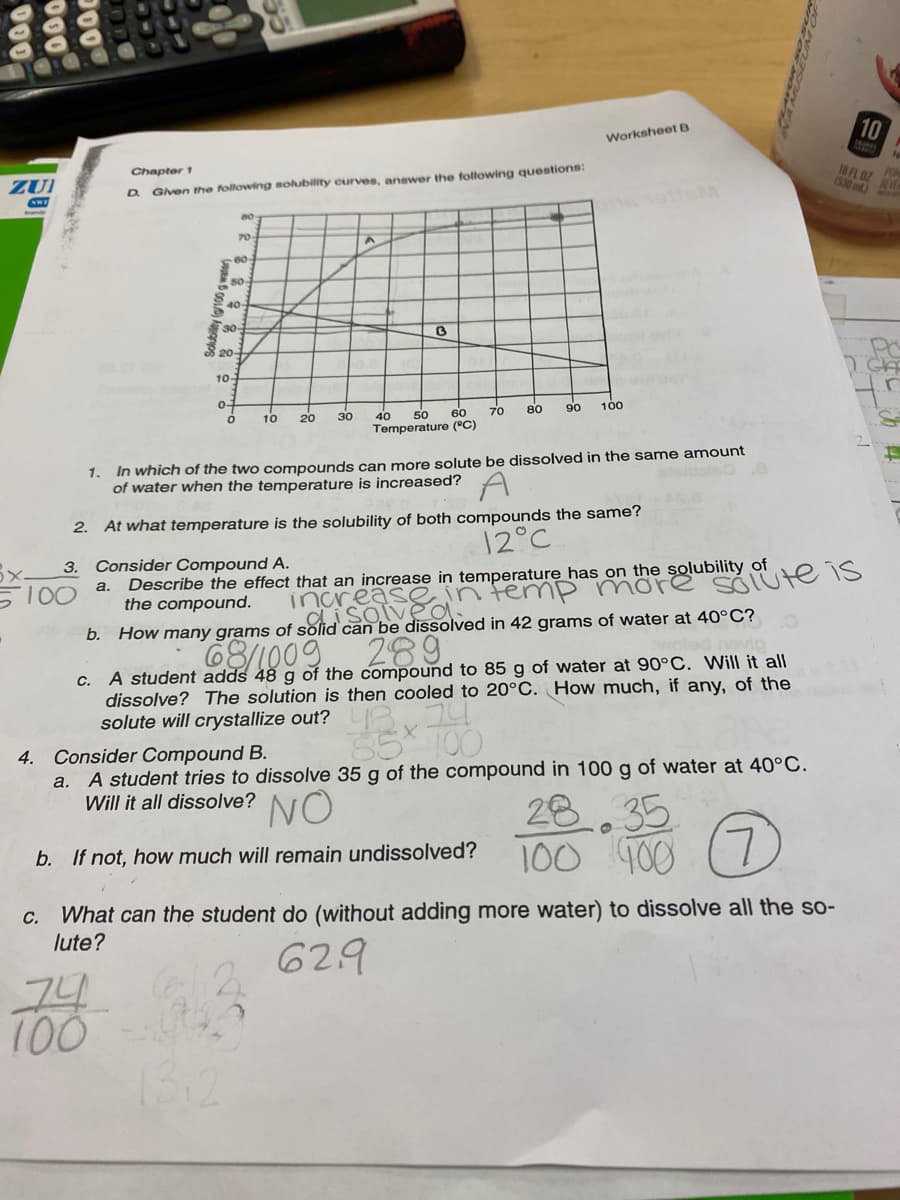
Chapter 1 18F (5:30 D Gven the following sohubility curves, anewer the following questions: 70 80 40 30- 20- 10 0- 10 20 30 50 60 70 80 90 100 40 Temperature ("C) 1. In which of the two compounds can more solute be dissolved in the same amount of water when the temperature is increased? A 2. At what temperature is the solubility of both compounds the same? 12°C 3. Consider Compound A. 100 Describe the effect that an increase in temperature has on the solubility of the compound. a. increase in temp more disolveO b. How many grams of solid can be dissolved in 42 grams of water at 40°C? 289 c. A student adds 48 g of the compound to 85 g of water at 90°C. Will it all dissolve? The solution is then cooled to 20°C. How much, if any, of the solute will crystallize out? LA
Chapter 1 18F (5:30 D Gven the following sohubility curves, anewer the following questions: 70 80 40 30- 20- 10 0- 10 20 30 50 60 70 80 90 100 40 Temperature ("C) 1. In which of the two compounds can more solute be dissolved in the same amount of water when the temperature is increased? A 2. At what temperature is the solubility of both compounds the same? 12°C 3. Consider Compound A. 100 Describe the effect that an increase in temperature has on the solubility of the compound. a. increase in temp more disolveO b. How many grams of solid can be dissolved in 42 grams of water at 40°C? 289 c. A student adds 48 g of the compound to 85 g of water at 90°C. Will it all dissolve? The solution is then cooled to 20°C. How much, if any, of the solute will crystallize out? LA
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 5ALQ: You have read that adding a solute to a solvent can both increase the boiling point and decrease the...
Related questions
Question
Transcribed Image Text:10
Worksheet B
Chapter 1
18 FL 0Z
POM
(530 m)
ZUI
Given the following soubility curves, answer the following questions:
D.
70
60
40-
30-
S 20-
10-
0-
70
80
90
100
30
60
40
Temperature (°C)
10
20
50
In which of the two compounds can more solute be dissolved in the same amount
of water when the temperature is increased?
1.
2. At what temperature is the solubility of both compounds the same?
12°C
Describe the effect that an increase in temperature has on the solubility of
the compound.
3. Consider Compound A.
2areassan temp more
68/1009.
SOIUte is
a.
S100
inca
can be dissolved in 42 grams of water at 40°C?
289
A student adds 48 g of the compound to 85 g of water at 90°C. Will it all
dissolve? The solution is then cooled to 20°C. How much, if any, of the
C.
solute will crystallize out?
35* 100
4. Consider Compound B.
a. A student tries to dissolve 35 g of the compound in 100 g of water at 40°C.
Will it all dissolve?
NO
28
35
100 400 (7)
L.
b. If not, how much will remain undissolved?
What can the student do (without adding more water) to dissolve all the so-
lute?
C.
629
74
100
13:2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning