Assuming that the yield of your amidation reaction was 100%, how many mole equivalents of diethylamine should be used in the second step of this synthesis? Why do you think the experiment is designed using this amount of diethylamine? Hint: what role(s) does diethylamine play in the reaction?
Assuming that the yield of your amidation reaction was 100%, how many mole equivalents of diethylamine should be used in the second step of this synthesis? Why do you think the experiment is designed using this amount of diethylamine? Hint: what role(s) does diethylamine play in the reaction?
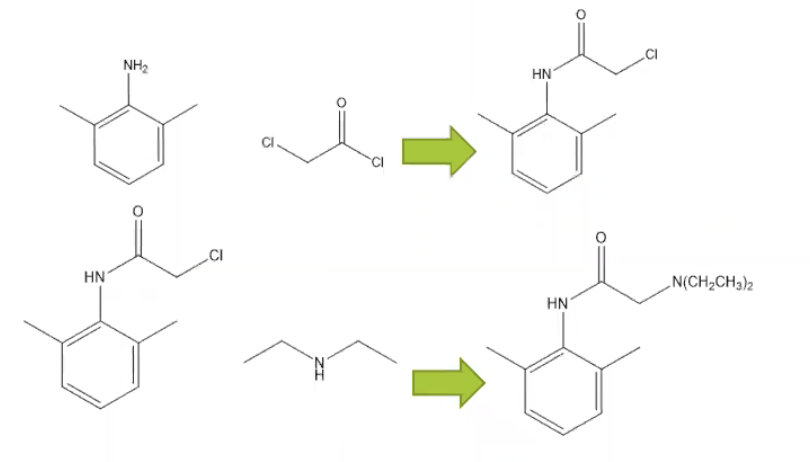
ATTACHED IS THE FULL REACTION
PROCEDURE
Synthesis of Amide 2
To a 125 mL Erlenmeyer flask containing a magnetic stirbar was added 2,6- dimethylaniline (1, 3.0 mL), followed by acetic acid (20 mL). The mixture was stirred until homogeneous and then chloroacetyl chloride (2.0 mL) was added in one portion. Immediately after the addition of chloroacetyl chloride, a concentrated solution of sodium acetate (25 mL) was added in one portion. The mixture was stirred for about five minutes and then DI water (65 mL) was added directly to the reaction mixture in one portion.
Synthesis of Lidocaine
The sample of 2 obtained in the previous step was added to a 50 mL round-bottom flask equipped with a couple of boiling chips, followed by toluene (25 mL) and then diethylamine (7.5 mL). The flask was fitted with a water-cooled condenser and the reaction mixture was refluxed for 80 minutes. The reaction mixture was then cooled to room temperature, transferred to a separatory funnel, and washed four times with DI water (50 mL per wash). The organic layer was then extracted with 3 M aqueous HCl (20 mL). The acidic aqueous layer was then transferred to a clean Erlenmeyer flask and cooled in an ice-water bath. Once the mixture was ~10 °C, the mixture was basified by portionwise2 addition of 2 M aqueous NaOH so that the temperature of the mixture did not exceed 20 °C. The resulting precipitate was collected by vacuum filtration, washed with DI water (50 mL), and then dried on the filter.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps









