Here's a one-batch sample of Just Lemons lemonade production. Determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. Hint: Complete stoichiometry calculations for each ingredient to determine the theoretical yield. Complete a limiting reactant-to-excess reactant calculation for both excess ingredients. Water Sugar Lemon Juice Lemonade Percent Leftover Yield Ingredients 946.36 g 196.86 g 193.37 g 719.84 g Sugar and water Just Lemons Lemonade Recipe Equation: 2 water + sugar + lemon juice 4 lemonade Mole conversion factors: BA..... 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 198 g 1 mole of lemon juice = 1 cup 229.96 g 1 mole of lemonade 1 cup 225.285 g Show your calculations below.
Here's a one-batch sample of Just Lemons lemonade production. Determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. Hint: Complete stoichiometry calculations for each ingredient to determine the theoretical yield. Complete a limiting reactant-to-excess reactant calculation for both excess ingredients. Water Sugar Lemon Juice Lemonade Percent Leftover Yield Ingredients 946.36 g 196.86 g 193.37 g 719.84 g Sugar and water Just Lemons Lemonade Recipe Equation: 2 water + sugar + lemon juice 4 lemonade Mole conversion factors: BA..... 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 198 g 1 mole of lemon juice = 1 cup 229.96 g 1 mole of lemonade 1 cup 225.285 g Show your calculations below.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 1STP
Related questions
Question
100%
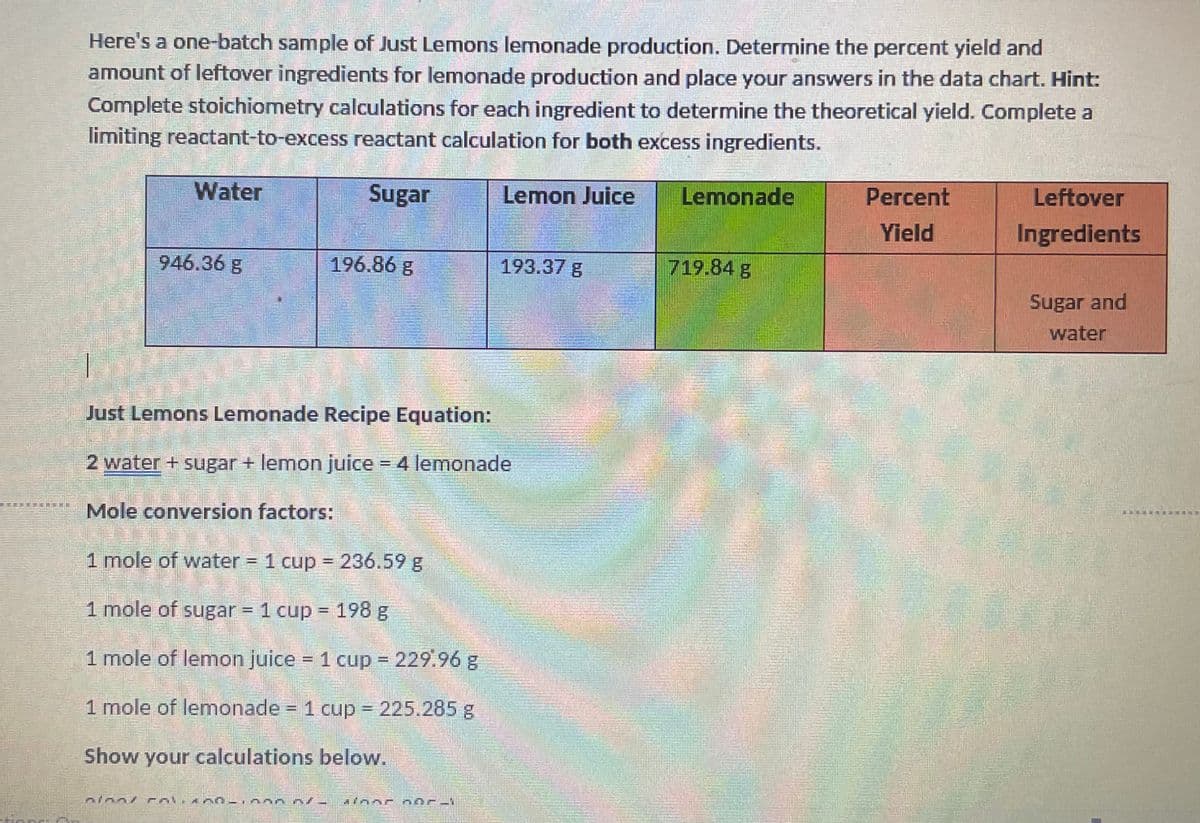
Transcribed Image Text:Here's a one-batch sample of Just Lemons lemonade production. Determine the percent yield and
amount of leftover ingredients for lemonade production and place your answers in the data chart. Hint:
Complete stoichiometry calculations for each ingredient to determine the theoretical yield. Complete a
limiting reactant-to-excess reactant calculation for both excess ingredients.
Water
Sugar
Lemon Juice
Lemonade
Percent
Leftover
Yield
Ingredients
946.36 g
196.86 g
193.37 g
719.84 g
Sugar and
water
Just Lemons Lemonade Recipe Equation:
2 water + sugar + lemon juice 4 lemonade
Mole conversion factors:
1 mole of water = 1 cup = 236.59 g
%3!
1 mole of sugar = 1 cup = 198 g
%3D
1 mole of lemon juice = 1 cup 229.96 g
1 mole of lemonade - 1 cup 225.285g
Show your calculations below.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning