Assuming you started with 3.23 g of sodium bicarbonate and your actual yield of solid product is 1.20g, which reaction is more likely to be the correct one?
Assuming you started with 3.23 g of sodium bicarbonate and your actual yield of solid product is 1.20g, which reaction is more likely to be the correct one?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 156QRT: Ethanol, C2H5OH, is a gasoline additive that can be produced by fermentation of glucose....
Related questions
Question
Assuming you started with 3.23 g of sodium bicarbonate and your actual yield of solid product is 1.20g, which reaction is more likely to be the correct one?
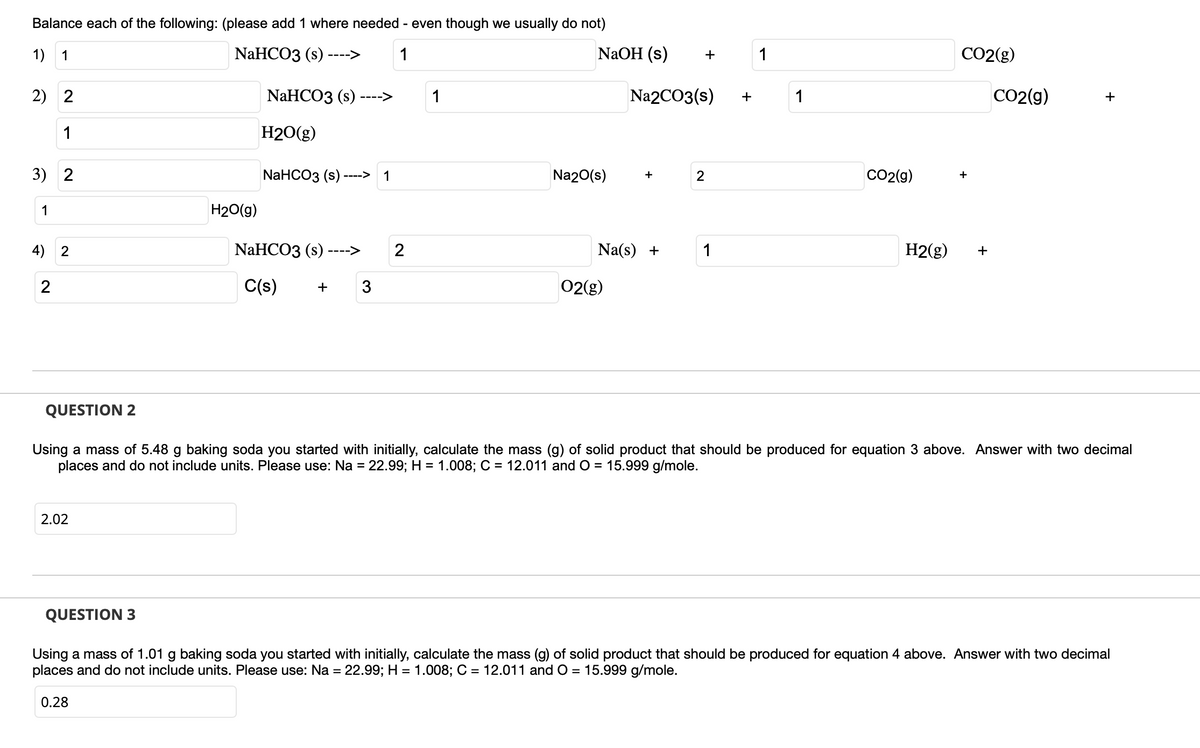
Transcribed Image Text:Balance each of the following: (please add 1 where needed - even though we usually do not)
1) 1
NaHCO3 (s) ---->
1
2) 2
NaHCO3 (s)
->
1
1
H2O(g)
3) 2
NaHCO3 (s)-
1
1
NaHCO3 (s) ----> 2
1
H2(g)
4) 2
2
C(s)
+ 3
02(g)
QUESTION 2
Using a mass of 5.48 g baking soda you started with initially, calculate the mass (g) of solid product that should be produced for equation 3 above. Answer with two decimal
places and do not include units. Please use: Na = 22.99; H = 1.008; C = 12.011 and O = 15.999 g/mole.
2.02
QUESTION 3
Using a mass of 1.01 g baking soda you started with initially, calculate the mass (g) of solid product that should be produced for equation 4 above. Answer with two decimal
places and do not include units. Please use: Na = 22.99; H = 1.008; C = 12.011 and O = 15.999 g/mole.
0.28
H₂O(g)
NaOH (s)
+
Na2CO3(s)
+
2
Na(s) +
Na2O(s)
+
1
1
CO2(g)
CO2(g)
+
CO2(g)
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning