At 25°C and 1.000 atm, methane gas (CH4) has a mean free path of 5.33×10-8 m. a-Calculate the effective hard-sphere diameter of methane molecules b- Calculate the number of collisions per second Za undergone by one methane molecule at this temperature and pressure c-Calculate the total number of collisions per cubic meter per second ZAA in methane gas at this temperature and pressure
At 25°C and 1.000 atm, methane gas (CH4) has a mean free path of 5.33×10-8 m. a-Calculate the effective hard-sphere diameter of methane molecules b- Calculate the number of collisions per second Za undergone by one methane molecule at this temperature and pressure c-Calculate the total number of collisions per cubic meter per second ZAA in methane gas at this temperature and pressure
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.94PAE: 94 Mining engineers often have to deal with gases when planning for the excavation of coal. Some of...
Related questions
Question
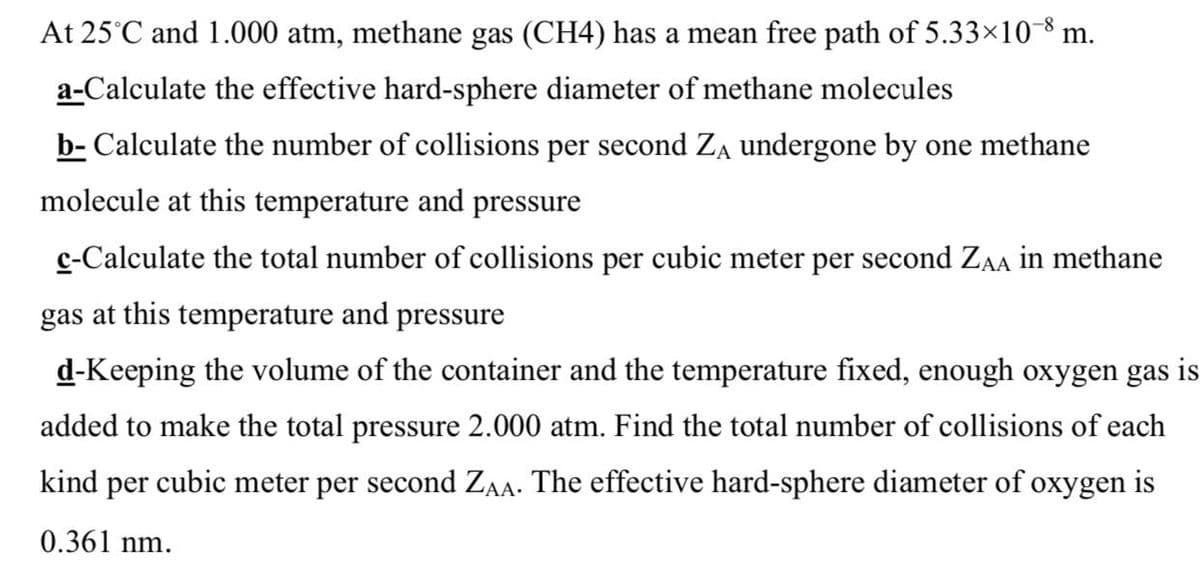
Transcribed Image Text:At 25°C and 1.000 atm, methane gas (CH4) has a mean free path of 5.33×10-8 m.
a-Calculate the effective hard-sphere diameter of methane molecules
b- Calculate the number of collisions per second Za undergone by one methane
molecule at this temperature and pressure
c-Calculate the total number of collisions per cubic meter per second ZAA in methane
gas at this temperature and pressure
d-Keeping the volume of the container and the temperature fixed, enough oxygen gas is
added to make the total pressure 2.000 atm. Find the total number of collisions of each
kind per cubic meter per second ZA. The effective hard-sphere diameter of oxygen is
0.361 nm.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
